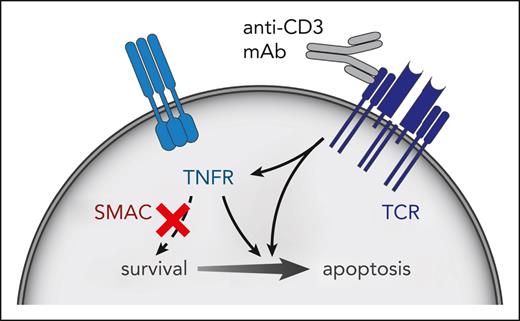
In this issue of Blood, Ávila Ávila et al1 report that T-cell acute lymphoblastic leukemia (T-ALL) cells overcome anti-CD3 monoclonal antibody (mAb)-directed therapy by inducing tumor necrosis factor receptor (TNFR) signaling, leading to activation of the NF-κB pathway (see figure). Combining teplizumab, an inhibitor of CD3, with etanercept, a decoy receptor for tumor necrosis factor-α (TNF-α), inhibited human T-ALL patient-derived xenograft (PDX) growth in mouse models, suggesting the importance of this pathway. Remarkably, coadministration of birinapant, which mimics the downstream regulator secondary mitochondrial-derived activator of caspases (SMAC), redirected prosurvival TNFR signaling into a parallel, apoptotic program and led to growth suppression and even complete cure in one human T-ALL model. This combination therapy demonstrates how an unwanted cell-signaling outcome can be rechanneled to improve overall treatment efficacy. Anti-CD3 combination therapy can be a new bridge to allogeneic stem cell transplant.
SMAC mimetic and anti-CD3 antibody activates cell death–promoting apoptotic signaling. This treatment inhibits T-ALL growth in mice.
SMAC mimetic and anti-CD3 antibody activates cell death–promoting apoptotic signaling. This treatment inhibits T-ALL growth in mice.
Patients with T-ALL who fail to achieve a remission at the end of induction chemotherapy or who relapse have poor long-term survival.2,3 Relapses can occur in 15% to 20% of children with T-ALL3 and a significantly higher percentage of adults. Progress is being made in new therapeutic approaches with nelarabine, an antimetabolite prodrug, alone or in combination with chemotherapy, inducing upward of 62% complete remissions.4 However, there remain patients who fail or do not respond to this therapy and require curative approaches.
In a set of articles published previously in Blood, Tran Quang and colleagues explored a novel approach to further T-ALL therapy by treatment with anti-CD3 antibodies to induce T-cell apoptosis.5,6 Most recently, they employed teplizumab, a fully humanized CD3ε-specific antibody with 2 point mutations in the Fc domain that reduce binding to Fc receptors and complement. Teplizumab has been used to treat T-cell–mediated allograft rejection in solid-organ transplant and, more recently, to halt the progression of type 1 diabetes. They also demonstrated that a combination with chemotherapy further suppressed tumor growth in preclinical studies.5 However, using human T-ALL PDX grown in mice, this therapy did not fully eliminate tumor growth.
Building on their previous work, Ávila et al have applied transcriptional profiling to anti-CD3 mAb-treated T-ALL cells to decipher which survival pathways are being activated by this therapy. Gene set enrichment analysis demonstrated a significant increase in T-cell receptor–induced genes related to apoptotic clonal selection of normal thymic progenitors, and, importantly, activation of the “TNF-α signaling via NF-κB” pathway. Induction of genes in this pathway were validated in 8 independent T-ALL PDXs.
TNFR signaling can lead to prosurvival or proapoptotic outcomes, depending on the activity of downstream components. To distinguish between these 2 possibilities, the authors coadministered anti-CD3 mAb and etanercept (a decoy receptor for TNF-α and lymphotoxin-α) and demonstrated enhanced efficacy of anti-CD3 therapy, indicating that TNFR signaling was prosurvival. Next, they blocked this survival signal using birinapant, a molecular mimic of the proapoptotic protein SMAC, which promotes TNFR-induced death signaling.7 Birinapant, currently in phase 2 clinical trials, and SMAC induce proteolysis of the prosurvival factors cellular inhibitor of apoptosis (cIAP) 1 and 2, which are E3 ligases that, when activated, play a role in stimulating NF-κB transcriptional activity. Inhibiting the cIAP signal leads to phosphorylation and activation of protein kinase receptor–interacting serine/threonine protein kinase 1 and induction of cell death.7 Birinapant did not show any single-agent efficacy in this study, in contrast to other published studies on treatment of solid tumors8,9 and B-ALL.10 When administered in combination with an anti-CD3 mAb, birinapant led to superior induction of apoptosis and clearance of T-ALL cells in the mouse xenotransplant model as compared with the anti-CD3 mAb alone.
The combination treatment was curative for 1 human PDX T-ALL donor patient tumor in mice, but in the other patient-derived tumors mice eventually died. Thus, important questions remain regarding how T-ALL tumor cells circumvent this new combined therapy. In addition, it is not yet known whether biomarkers exist that could predict both partial and complete response. Although birinapant is under investigation, the combination of etanercept and teplizumab, both approved by the US Food and Drug Administration, could be pursued in clinical trials. These therapies, although not curative, may provide a window of opportunity as an important bridge to allogeneic stem cell transplant. In addition, Ávila et al have also shown activity of anti-CD3 in combination with navitoclax, a BCL-2 (B-cell lymphoma 2), BCL-xL, and BCL-w inhibitor, suggesting that new combinations with tepluzimab could have a significant impact on outcome in T-ALL.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal