In this issue of Blood, Hayashi et al1 assessed the correlation of the level of measurable residual disease (MRD) at the end of induction (EOI) with event-free survival (EFS) in a subset of children and young adults (aged 1-30 years) with T-cell lymphoblastic lymphoma (T-LL, n = 86) treated in a phase 3 trial by the Children’s Oncology Group (COG). The group showed that patients with MRD < 0.1% (n = 75) at EOI had a better 4-year EFS vs those with MRD > 0.1% (n = 11). The authors suggest incorporating EOI MRD as an essential tool for prognostication of patients with T-LL.
T-LL is mostly seen in adolescents and young adults, has a male predominance, and usually presents with advanced stage III to IV disease; 90% of patients have a mediastinal mass, sometimes with concomitant pleural and pericardial effusions. Central nervous system (CNS) involvement is seen in 5% to 10% of cases, and diffuse adenopathy or other organs are involved in 70% of cases. Clinically, T-LL and T-cell acute lymphoblastic lymphoma (T-ALL) are separated by an arbitrary cut point of 25% bone marrow infiltration, although there are differences between these 2 diseases at a genetic level.2,3 T-LL shows a prevalence of 70% of the thymic subtype. Treatment should be based on ALL regimens.4 In adults, complete remission (CR) is achieved in 70% to 90% of cases in most studies, and the EFS probability is 60% to 70% at 5 years.5 Better results are observed in children, with EFS reaching 80% to 90%.6 In view of these results, hematopoietic stem cell transplantation is reserved for patients in second CR or refractory disease.
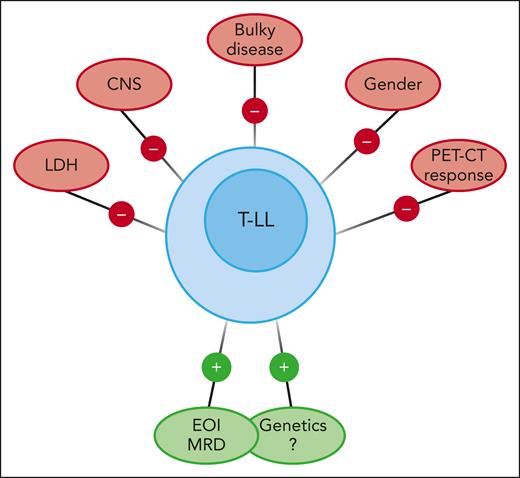
There are no universally accepted adverse prognostic factors for T-LL. They differ across trials, such as increased lactate dehydrogenase, CNS involvement, and genetic classification, among other factors.4 Traditional variables such as race, age, sex, stage, bulky disease, or radiologic response to therapy have failed to correlate with EFS in children and adolescents (see figure). The prognostic value of positron emission tomography–computed tomography (PET-CT) imaging in T-LL remains unclear.7 As MRD is prognostic for most hematologic malignancies, it seems logical to look for the prognostic value of MRD in the bone marrow at a relevant time point such as EOI. Hayashi et al demonstrated that MRD at EOI, assessed with flow cytometry with a validated sensitivity of 0.01%, is an independent risk factor for EFS regardless of the treatment arm in the AALL1231 randomized trial (COG study of modified augmented Berlin-Frankfurt-Münster backbone vs the same schedule with the addition of bortezomib during induction and delayed intensification).
Favorable and unfavorable prognostic factors in children and adolescents with T-LL.
Favorable and unfavorable prognostic factors in children and adolescents with T-LL.
Identification of the prognostic value of MRD represents a step forward in T-LL given the lack of consistently proven prognostic factors available to date. As the sample size in the present study was limited, the authors recognized that incorporation of MRD at the EOI in large clinical trials will be needed to definitively establish its future value in risk stratification. However, it seems logical to assume that this will be the case, as MRD is predictive in T- and B-cell precursor ALL.
Some questions arise from the results presented. (1) Is bone marrow involvement detectable by flow cytometry at diagnosis in all patients with T-LL? (2) Is the EOI MRD level correlated with the genetic or genomic abnormalities of T-LL,8,9 or can it be considered independently for prognosis assessment? (3) Could the cutoff level of 0.01% at EOI be more useful for prognosis than 0.1%? (This question was not fully addressed in the study by Hayashi et al probably due to the small sample size.) (4) Is the sensitivity level of 0.01% achieved by conventional flow cytometry sufficient? (5) Is EOI the only time point useful for prognostication, or do we need MRD assessment after consolidation as occurs in ALL? (6) Does the EOI MRD level correlate with the clearance of extramedullary disease assessed by PET-CT scan? And (7) does MRD have the same prognostic value after treatment for relapse?
The study by Hayashi et al has obvious clinical relevance. As T-LL and T-ALL are considered within the spectrum of the same disease from a therapeutic point of view, it seems logical to address the same questions of MRD as those used for T-ALL. Given the low frequency of T-LL, well-designed multicenter trials are necessary to achieve this objective.
Conflict-of-interest disclosure: J.-M.R. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal