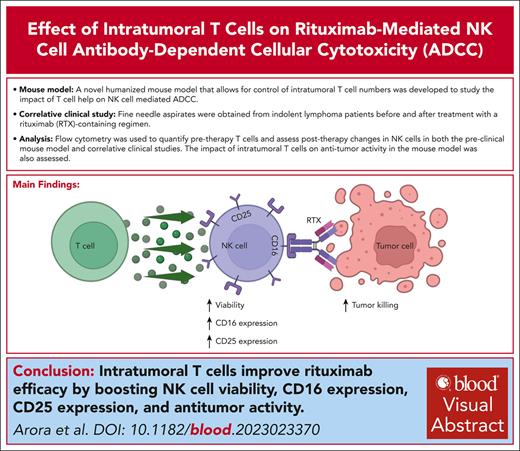
T-cell help in the TME enhances rituximab-mediated NK-cell viability, CD16 and CD25 expression, and antitumor response.
Visual Abstract
Rituximab (RTX) and other monoclonal antibodies (mAbs) that bind directly to malignant cells are of great clinical value but are not effective for all patients. A major mechanism of action of RTX is antibody-dependent cellular cytotoxicity (ADCC) mediated by natural killer (NK) cells. Prior in vitro studies in our laboratory demonstrated that T cells contribute to maintaining the viability and cytotoxic potential of NK cells activated by anti-CD20–coated target B cells. Here, we conducted studies using a novel mouse model and clinical correlative analysis to assess whether T-cell help contribute to RTX-mediated NK-cell ADCC in the tumor microenvironment (TME) in vivo. A humanized mouse model was developed using Raji lymphoma cells and normal donor peripheral blood mononuclear cells that allows for control of T-cell numbers in the lymphoma TME. In this model, NK-cell viability and CD16 and CD25 expression dropped after RTX in the absence of T cells but increased in the presence of T cells. RTX therapy was more effective when T cells were present and was ineffective when NK cells were depleted. In patients with indolent lymphoma, fine needle aspirates were obtained before and ∼1 week after treatment with a RTX-containing regimen. There was a strong correlation between CD4+ T cells as well as total T cells in the pretherapy TME and an increase in NK-cell CD16 and CD25 expression after RTX. We conclude that T-cell help in the TME enhances RTX-mediated NK-cell viability and ADCC.
Introduction
Monoclonal antibodies (mAbs) that mediate their antitumor effect by binding directly to cancer cells are a crucial component of therapy for various malignancies. The anti-CD20 mAb rituximab (RTX) is a mainstay treatment for B-cell malignancies. Although RTX is effective, up to 60% of treated patients develop resistance.1,2 The mechanisms behind response or resistance to RTX are incompletely understood. Proposed mechanisms of action for mAbs, including RTX, are antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity, antibody-dependent cellular phagocytosis (ADCP), and signaling-induced apoptosis, with the predominant mechanism varying based on the cancer and model studied.3-9 Strong, if indirect, evidence points toward ADCC mediated by human natural killer (NK) cells as being a key clinical mechanism of action for many mAbs including RTX.10-12 ADCC involves RTX binding to CD20 on tumor cells that triggers NK-cell activation via surface Fc receptors (FcgRIIIa/CD16) and leads to the release of cytotoxic granules and subsequent tumor cell death.13 Studies performed when single-agent RTX was used more commonly for follicular lymphoma showed that patients with a high-affinity FcgRIIIa polymorphism (CD16 158V) treated with single-agent RTX had better outcomes than those with the lower affinity polymorphism (CD16 158F).10,14 Subsequent clinical studies of RTX, including those in which RTX was combined with chemotherapy, failed to show a correlation between CD16 polymorphisms and response.15-18 Convincing data in mouse models suggest ADCP mediated by myeloid cells is central to the response to anti-CD20 mAb, but the importance of ADCP in the human system is less clear.8,9 Overall, these data suggest, but do not confirm, that ADCC mediated by NK cells via CD16 is an important clinical mechanism of action for RTX.
A T-cell signature indicating the presence of tumor-infiltrating T cells has been reported to correlate with improved outcomes in follicular lymphoma.19-21 We previously reported in vitro studies that provided a potential explanation for this finding. After activation by anti-CD20–coated target B cells, T cells contribute to maintaining NK-cell viability and expression of NK-cell CD16 and CD56, as well as the ability to mediate ADCC. This effect is largely dependent on CD4+ T cells, contact between the T cells and NK cells, and is mediated in large part by interleukin-2 (IL-2).22 Upon stimulation with IL-2, NK cells increase the surface expression of CD25 (the α chain of the high-affinity IL-2 receptor), enhancing their proliferation and cytotoxicity.23 Although in vitro studies have provided valuable insights into mAb-mediated ADCC, they may not fully represent the complex in vivo tumor microenvironment (TME). Investigation of this observation in syngeneic immunocompetent mouse models would be of limited value, given the major differences in NK-cell biology between humans and mice.24 Existing humanized mouse models lack NK-cell infiltration in the TME and pose challenges for TME control and modification.25 Here, we report the results of in vivo studies supporting the hypothesis that T-cell help contributes to RTX-mediated NK-cell ADCC in the TME. These studies include a novel humanized mouse model and clinical correlative analysis of involved node fine needle aspirates (FNAs) from patients with lymphoma treated with RTX. These results could have significant impact on our understanding of a previously unrecognized mechanism of resistance to RTX and point toward potential approaches to addressing that resistance.
Methods
Development of humanized mouse model
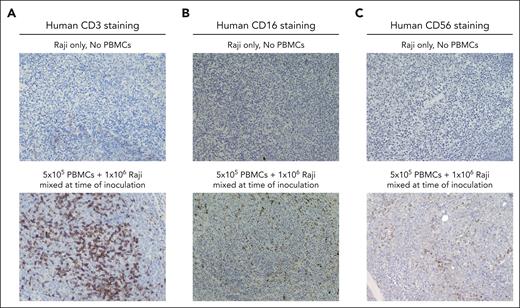
Mouse studies were approved and performed according to guidelines established by the University of Iowa Institutional Animal Care and Use Committee (IACUC). Female NOD CRISPR Prkdc Il2r gamma (NCG) triple immunodeficient mice (aged 42-48 days) and prequalified human peripheral blood mononuclear cells (PBMCs) were obtained from Charles River.26 For pilot studies, 5 groups of 3 mice each were evaluated. All mice were inoculated with 1 × 106 Raji lymphoma cells (CCL-86; American Type Culture Collection) subcutaneously in the flank. Group 1 received Raji cells alone without any PBMCs. Groups 2 and 3 were inoculated with Raji cells mixed with 1 × 105 PBMCs and 5 × 105 PBMCs, respectively, subcutaneously in the flank. Mice in group 4 were systemically injected via retro-orbital sinus with 1 × 107 PBMCs 10 days before Raji inoculation. For group 5, a total of 1 × 107 PBMCs were systemically injected 12 days after Raji inoculation. Once palpable tumors developed, serial FNAs were obtained twice a week and analyzed by flow cytometry. Tumor dimensions of length, width, and height were measured twice weekly with a caliper, and the tumor volume was calculated using the formula for the volume of an ellipsoid: tumor volume (mm3) = π/6 (length × width × height). Mice were euthanized when the tumor diameter exceeded 20 mm in any dimension. Tumor tissues were harvested between 25 and 27 days after tumor inoculation, and slides were prepared for immunohistochemistry (IHC) staining for T cells (CD3) and NK cells (CD16 and CD56), in collaboration with the University of Iowa Comparative Pathology Laboratory. Images were taken using an Eclipse Ci (Nikon) microscope at 20× magnification.
Evaluation of the impact of modified T-cell numbers in the TME on NK-cell phenotype in a humanized mouse model
PBMCs from a qualified donor were divided into 2 aliquots. T cells were depleted from 1 aliquot and isolated from the other aliquot using magnetic-activated cell sorting negative selection (Miltenyi Biotec). A cell mixture was produced containing 1 × 106 Raji cells and 5 × 105 T-cell–depleted PBMCs to which varying numbers of isolated CD3+ T cells (0%, 5%, and 20%) from the same donor were added back. These percentages were based on in vitro studies showing >10% T cells provide adequate T-cell help. Thus, the inoculum had a constant number of Raji cells and non-T PBMCs but varying numbers of T cells, namely 0% T cells (0 T cells per mouse), 5% T cells (2.5 × 104 T cells per mouse), or 20% T cells (1 × 105 T cells per mouse). This mixture was inoculated into the flank of NCG mice on day 0. RTX and trastuzumab (TRA) were obtained from discard clinical vials through the Holden Comprehensive Cancer Center. Once the tumors became palpable (on day 20), pretreatment FNAs were obtained, and mice were treated intraperitoneally (IP) with 30 mg/kg mAb consisting of either RTX (10 mice per group) or isotype-matched mAb TRA (5 mice per group). Posttreatment FNAs were obtained 4 days after mAb therapy.
Assessing the impact of intratumoral T cells on NK-cell–mediated therapeutic response to RTX in a humanized mouse model
Mice were inoculated with a mixture of Raji cells and PBMCs. T cells were depleted from the PBMCs, and controlled numbers of T cells were added back, or CD56+ cells were depleted before PBMCs were mixed with Raji cells and inoculated into mice. Luciferase-expressing Raji-Luc cells (CCL-86-LUC2; American Type Culture Collection) were used to allow for the quantification of tumor burden by bioluminescent imaging. Mice were administered 30 mg/kg RTX or TRA IP beginning on day 7 after tumor injection, with treatment repeated weekly. In vivo bioluminescent imaging was done biweekly, starting from day 1. A freshly prepared solution of luciferin at a concentration of 15 mg/mL was injected IP 10 minutes before imaging and scanned for bioluminescence using an in vivo imaging system (IVIS) (Lumina S5; Revvity). Regions of interest encompassing the tumor were delineated, and total flux (p/s) was assessed to reflect tumor burden.
Correlative clinical study
Patients with indolent B-cell lymphoma scheduled to receive a combination of RTX and bendamustine per standard of care27 were enrolled on an institutional review board–approved clinical study (202012153). After written informed consent, a pretreatment FNA was obtained. Patients received therapy per standard protocol, and a second FNA was performed 1 to 2 weeks later. Standard of care therapy was provided for the remainder of the treatment course.
Flow cytometric evaluation
FNAs from both humanized mice and patients in clinical trial were stained using the same flow cytometric panel (supplemental Table 1, available on the Blood website). A 5-laser spectral flow cytometer (Cytek Aurora) was used for data acquisition and analyzed with FlowJo v10.8 software. The gating strategy included live CD45+ cell selection, followed by singlet cell gating and subsequent identification of specific immune cell populations based on their markers. Cell frequency (% cells) of the single live CD45+ cell population and median fluorescence intensity for specific surface markers were calculated.
Statistical analysis
Humanized mouse model data were analyzed using 1-way analysis of variance with Tukey multiple comparison tests. Estimated means from the analysis were plotted along with standard deviation. Survival data were plotted with the Kaplan-Meier method and analyzed using the log-rank test. Clinical data were summarized descriptively with means, standard deviations, medians, and ranges for numeric characteristics (supplemental Table 2). Associations between pretherapy T-cell counts and fold changes in NK cells were estimated and tested with Spearman rank correlation. All statistical testing was 2-sided and assessed for significance at the 5% level. All statistical analyses were conducted in GraphPad Prism version 9.4.1 and R.
The described clinical trial was approved by the University of Iowa Institutional Review Board (202012153), and animal studies were approved by the University of Iowa IACUC (1011236).
Results
Mixing PBMCs with tumor cells at the time of tumor inoculation allows for the control of T cells and NK cells in the TME of resulting xenograft tumors
NCG mice were inoculated with Raji cells and PBMCs as described in “Methods.” The presence of PBMCs had no discernable effect on tumor growth in the absence of therapy (supplemental Figure 1). As shown in Table 1, flow cytometric evaluation of FNAs obtained from tumors 17 to 18 days after tumor inoculation revealed engraftment of T cells in TME regardless of whether PBMCs were given locally or systemically. NK cells were found in the TME of emerging tumors in mice when PBMCs were mixed with Raji cells and coinjected, however, very few NK cells were found in the tumors of mice that had been systemically injected with PBMCs. In mice coinjected with Raji and PBMCs subcutaneously, the frequency of T cells and NK cells in the TME correlated with the number of PBMCs in the inoculum (group 2 vs group 3). Serial FNAs showed relatively consistent total CD3+ T-cell numbers over time. However, some decrease in CD4+ T cells and NK cells was observed between 17 and 24 days after tumor inoculation. Few CD14+ myeloid cells were present in the FNAs (supplemental Figure 2). Immune cell distribution within the tumor was relatively uniform as indicated by IHC of tumors harvested 27 days after tumor inoculation (Figure 1). Overall, these studies demonstrated that local injection of PBMCs mixed with Raji tumor cells can be used to adjust the number of T cells and NK cells within the TME, with the number of CD4+ T cells and NK cells dropping beginning ∼3 weeks after inoculation.
Development of humanized mouse model with controllable numbers of NK cells and T cells in TME
| Group . | Description . | Cells in inoculum . | Cells in tumor FNA 17-18 d after tumor inoculation . | ||
|---|---|---|---|---|---|
| PBMCs . | Raji . | % T cells∗ . | % NK cells∗ . | ||
| 1 | No PBMCs | — | 1 × 106 | 0 ± 0 | 0 ± 0 |
| 2 | Locally injected PBMCs-Raji mixed at time of inoculation | 1 × 105 | 1 × 106 | 8.7 ± 0.1 | 1.5 ± 0.6 |
| 3 | Locally injected PBMCs-Raji mixed at time of inoculation | 5 × 105 | 1 × 106 | 24.4 ± 15.2 | 4.4 ± 2.5 |
| 4 | Systemically injected PBMCs 10 d before Raji inoculation | 1 × 107 | 1 × 106 | 25.2 ± 6.9 | 0.4 ± 0.3 |
| 5 | Systemically injected PBMCs 12 d after Raji inoculation | 1 × 107 | 1 × 106 | 14.6 ± 11 | 1 ± 0.1 |
| Group . | Description . | Cells in inoculum . | Cells in tumor FNA 17-18 d after tumor inoculation . | ||
|---|---|---|---|---|---|
| PBMCs . | Raji . | % T cells∗ . | % NK cells∗ . | ||
| 1 | No PBMCs | — | 1 × 106 | 0 ± 0 | 0 ± 0 |
| 2 | Locally injected PBMCs-Raji mixed at time of inoculation | 1 × 105 | 1 × 106 | 8.7 ± 0.1 | 1.5 ± 0.6 |
| 3 | Locally injected PBMCs-Raji mixed at time of inoculation | 5 × 105 | 1 × 106 | 24.4 ± 15.2 | 4.4 ± 2.5 |
| 4 | Systemically injected PBMCs 10 d before Raji inoculation | 1 × 107 | 1 × 106 | 25.2 ± 6.9 | 0.4 ± 0.3 |
| 5 | Systemically injected PBMCs 12 d after Raji inoculation | 1 × 107 | 1 × 106 | 14.6 ± 11 | 1 ± 0.1 |
Mean ± standard deviation.
Emerging tumors exhibit uniform distribution of T cells and NK cells in the TME. Humanized mouse xenograft tumors were harvested 27 days after tumor inoculation. IHC staining for hematoxylin and eosin, along with specific markers for CD3 (A), CD16 (B), and CD56 (C), was used to assess the distribution of immune cells within the TME.
Emerging tumors exhibit uniform distribution of T cells and NK cells in the TME. Humanized mouse xenograft tumors were harvested 27 days after tumor inoculation. IHC staining for hematoxylin and eosin, along with specific markers for CD3 (A), CD16 (B), and CD56 (C), was used to assess the distribution of immune cells within the TME.
T cells in the TME enhance NK-cell viability and CD16 and CD25 expression after RTX therapy
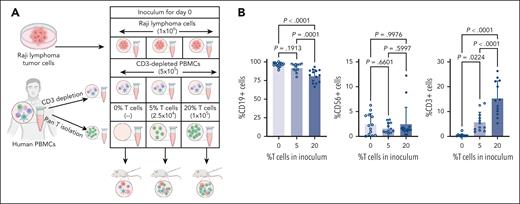
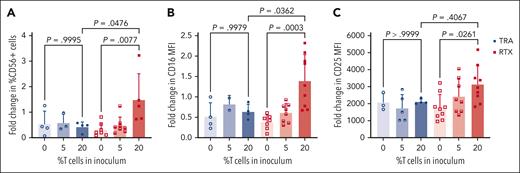
Using this model of inoculating mice with a mixture of PBMCs and tumor cells, we assessed the impact of T cells in the TME on NK cells after mAb therapy. This was done as described in “Methods” by keeping the number of Raji tumor cells and CD3-depleted PBMCs in the inoculum constant but varying the percentage of autologous T cells (0%, 5%, and 20% T cells) (Figure 2A). When tumors emerged 20 days after inoculation, pretreatment FNAs were obtained. Flow cytometric analysis of pretreatment FNAs confirmed that T-cell numbers adjusted in the initial inoculum resulted in varying T-cell numbers in emerging tumors. The number of T cells in the inoculum had no detectable impact on the ratio of tumor cells or NK cells (Figure 2B). Mice were then treated with a single dose of RTX or control mAb (TRA) IP, and second FNAs were obtained 4 days later. As illustrated in Figure 3A, mice that had 20% T cells in the tumor inoculum and were treated with RTX had increased numbers of NK cells in the post–RTX treatment TME. NK-cell numbers decreased in RTX-treated mice with lower numbers of T cells (0% or 5%) and in mice treated with TRA, irrespective of T cells. Thus, T cells are essential to maintain the viability of RTX–activated NK cells in the TME. Phenotypically, an increase in the expression of CD16 and CD25 by NK cells was only seen in mice bearing tumors with larger numbers of T cells treated with RTX (Figure 3B-C). Tumors contained very few myeloid cells. Most of the RTX–activated CD16+ cells coexpressed CD56 but not CD14 or CD3, indicating these cells were NK cells and not myeloid cells or gamma-delta T cells (supplemental Figure 3). Thus, this model is not helpful in assessing the role of myeloid cells or gamma-delta cells in the TME.
Adjusting T-cell numbers in the inoculum results in varying T-cell numbers in the emerging tumors but does not have a detectable impact on fractions of malignant cells or NK cells. (A) Schematic diagram of a humanized mouse model in which mice were inoculated on day 0 with consistent numbers of Raji tumor cells and T-cell–depleted PBMCs but varying numbers of T cells. (B) Flow cytometric analysis of FNAs obtained from mouse xenografts 20 days after inoculation and evaluated for percent of cells that are CD19+, CD56+, or CD3+ (n = 12-15 mice per group).
Adjusting T-cell numbers in the inoculum results in varying T-cell numbers in the emerging tumors but does not have a detectable impact on fractions of malignant cells or NK cells. (A) Schematic diagram of a humanized mouse model in which mice were inoculated on day 0 with consistent numbers of Raji tumor cells and T-cell–depleted PBMCs but varying numbers of T cells. (B) Flow cytometric analysis of FNAs obtained from mouse xenografts 20 days after inoculation and evaluated for percent of cells that are CD19+, CD56+, or CD3+ (n = 12-15 mice per group).
Humanized mouse model: T cells in the pretherapy TME increase NK-cell viability and CD16 and CD25 expression after RTX therapy. Flow cytometric analysis of intratumoral NK cells before and after RTX therapy. Mice were inoculated on day 0 with Raji tumor cells mixed with PBMCs containing varying percentages of T cells. After tumors developed on day 20, FNAs were obtained, and mice were treated systemically with RTX (or TRA as control). On day 24 (4 days after mAb dose), a second FNAs were obtained and before to after therapy changes in NK-cell numbers (A), NK-cell CD16 expression (B), and NK-cell CD25 expression (C) were determined.
Humanized mouse model: T cells in the pretherapy TME increase NK-cell viability and CD16 and CD25 expression after RTX therapy. Flow cytometric analysis of intratumoral NK cells before and after RTX therapy. Mice were inoculated on day 0 with Raji tumor cells mixed with PBMCs containing varying percentages of T cells. After tumors developed on day 20, FNAs were obtained, and mice were treated systemically with RTX (or TRA as control). On day 24 (4 days after mAb dose), a second FNAs were obtained and before to after therapy changes in NK-cell numbers (A), NK-cell CD16 expression (B), and NK-cell CD25 expression (C) were determined.
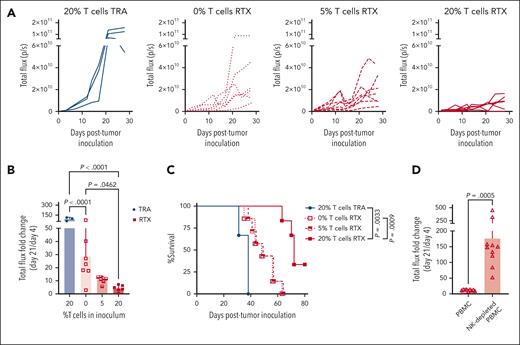
Intratumoral T cells enhance the therapeutic efficacy of RTX
Treatment with mAb on day 20, when large tumors had emerged, was used to assess changes in the phenotype of NK cells within the TME in response to therapy, but tumors were too large at the time of treatment to detect a meaningful antitumor response. The study was repeated with mAb treatment beginning 7 days after tumor and PBMC inoculation to avoid the need to euthanize mice with large tumors before the therapeutic impact of therapy could be observed. Groups were similar to those described for the phenotypic studies. Luciferase-expressing Raji cells were used to allow for the quantification of tumor burden using IVIS. As illustrated in Figure 4, RTX was most effective in mice with tumors containing 20% T cells, whereas TRA had no detectable antitumor effect even when T cells were present. Similar results demonstrating that T cells enhance RTX efficacy were found when antitumor response was determined using IVIS (Figure 4A-B; supplemental Figure 4A), tumor size based on caliper measurements (supplemental Figure 5A-B), or survival (Figure 4C). RTX was ineffective when PBMCs were depleted of NK cells before the mixing of PBMCs with Raji cells at the time of tumor inoculation (Figure 4D; supplemental Figures 4B and 5C). All mice depleted of NK cells and treated with RTX were euthanized between days 24 and 30 due to uncontrolled tumor growth similar to the tumor growth and survival of untreated mice in prior experiments. These data provide further evidence that NK cells play a central role in the antitumor activity of RTX in this mouse model.
Intratumoral T cells enhance NK-cell–mediated RTX efficacy. (A-C) Mice were inoculated with luciferase-expressing Raji-Luc cells mixed with PBMCs containing varying percentages of T cells, on day 0. Weekly treatment with RTX or TRA was started on day 7. (A) Spider plot of total flux determined using bioluminescent imaging. (B) Changes in tumor flux after treatment compared with that of before treatment (day 21 vs day 4). (C) Kaplan-Meier curve of survival. Mice were euthanized when tumors exceeded 20 mm in any dimension. (D) In a separate experiment, mice were inoculated with Raji-Luc mixed with either PBMCs or CD56-depleted PBMCs on day 0, followed by weekly RTX treatment starting on day 7. Bioluminescent imaging was used to determine changes in tumor flux after treatment compared with before treatment (day 21 vs day 4).
Intratumoral T cells enhance NK-cell–mediated RTX efficacy. (A-C) Mice were inoculated with luciferase-expressing Raji-Luc cells mixed with PBMCs containing varying percentages of T cells, on day 0. Weekly treatment with RTX or TRA was started on day 7. (A) Spider plot of total flux determined using bioluminescent imaging. (B) Changes in tumor flux after treatment compared with that of before treatment (day 21 vs day 4). (C) Kaplan-Meier curve of survival. Mice were euthanized when tumors exceeded 20 mm in any dimension. (D) In a separate experiment, mice were inoculated with Raji-Luc mixed with either PBMCs or CD56-depleted PBMCs on day 0, followed by weekly RTX treatment starting on day 7. Bioluminescent imaging was used to determine changes in tumor flux after treatment compared with before treatment (day 21 vs day 4).
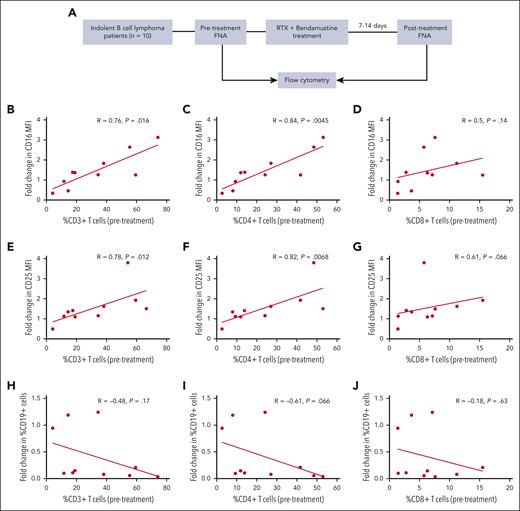
The fraction of intratumoral T cells, particularly CD4+ T cells, correlates with enhanced NK-cell CD16 expression after clinical treatment with an RTX-containing regimen
Correlative clinical studies were done to assess whether results after clinical therapy with RTX are consistent with the phenotypic findings presented in the xenograft model and previously published in vitro results.22 As described in “Methods,” participants included patients with indolent B-cell lymphoma scheduled to receive therapy with RTX and bendamustine based on clinical indications. FNAs were obtained before therapy and 1 to 2 weeks after the first cycle of therapy (see schema in Figure 5A). Flow cytometric evaluation demonstrated a strong correlation between the frequency of CD4+ T cells in the pretherapy TME and an increase in CD16 expression of NK cells after treatment (P = .0045) (Figure 5C). Changes in CD25 expression by NK cells after RTX therapy also correlated with the frequency of CD4+ T cells in the pretherapy FNA (P = .0068) (Figure 5F). Similar correlations with changes in CD16 and CD25 expression were seen with pretherapy CD3+ T cells (Figure 5B,E) but not with CD8+ T cells (Figure 5D,G), consistent with the in vitro findings indicating CD4+ T cells provide most of the T-cell help to RTX–activated NK cells. Consistent with the results in the xenograft model, patients with <10% CD4+ T cells failed to exhibit an increase in CD16 expression on NK cells after RTX treatment. A majority of CD16+ cells in the posttherapy FNAs coexpressed CD56 but not CD14 or CD3 and therefore were NK cells and not myeloid or gamma-delta T cells (supplemental Figure 3). All but 1 patient had a clinical response to therapy, thus it was not possible in this small study to assess whether intratumoral T-cell frequency affects the clinical therapeutic response to an RTX-containing regimen. There was a nonsignificant trend (P = .066) when assessing whether high pretherapy CD4+ cell levels correlate with a reduction in CD19+ cell frequency in the posttherapy FNA (Figure 5I). Less of an effect was seen with the overall CD3+ cells or CD8+ cells (Figure 5H,J). One patient had 24% T cells in the TME but no detectable reduction in CD19+ cells. This patient had an unusually high number of neutrophils (21.5%; typical frequency, 0.5%-10%) in the pretherapy FNA, suggesting a different biology. The number of patients in this study was limited, and it was not designed or powered to assess the correlation between the presence of T cells in the TME and clinical outcomes. However, it did demonstrate that CD4+ T cells in the pretherapy TME correlate with an increase in NK-cell CD16 and CD25 expression by NK cells after RTX treatment, which is consistent with in vitro and preclinical animal model results.
Correlative clinical trial results: CD4+ T cells in the pretherapy TME correlate with an increase in NK-cell CD16 and CD25 expression after RTX-containing therapy. (A) Clinical trial schema: patients (n = 10) with indolent B-cell lymphoma were treated with RTX and bendamustine based on clinical indications. FNAs from involved nodes were obtained before and 7 to 14 days after the first course of treatment. Pretherapy frequency of CD3+, CD4+, and CD8+ cells in the TME was correlated with pretherapy to posttherapy changes in expression intensity by NK cells of CD16 (B-D) and CD25 (E-G) as well as CD19 cell frequency (H-J).
Correlative clinical trial results: CD4+ T cells in the pretherapy TME correlate with an increase in NK-cell CD16 and CD25 expression after RTX-containing therapy. (A) Clinical trial schema: patients (n = 10) with indolent B-cell lymphoma were treated with RTX and bendamustine based on clinical indications. FNAs from involved nodes were obtained before and 7 to 14 days after the first course of treatment. Pretherapy frequency of CD3+, CD4+, and CD8+ cells in the TME was correlated with pretherapy to posttherapy changes in expression intensity by NK cells of CD16 (B-D) and CD25 (E-G) as well as CD19 cell frequency (H-J).
Discussion
mAbs that bind directly to malignant cells, including RTX, have been mainstays of cancer therapy for >2 decades, yet our understanding of the mechanisms of response or resistance to therapy with such mAbs is still incomplete. We previously reported in vitro studies suggesting T-cell help contributes to the efficacy of RTX (and other mAbs) by sustaining NK-cell viability and enhancing NK-cell–mediated ADCC.22 Such in vitro studies offered us the ability to control conditions precisely and analyze a large number of variables. However, in vitro cultures vary from in vivo conditions in multiple additional ways including, but not limited to, lack of the architecture of the TME, lack of stromal cells, and the metabolic milieu. Cell lines and immune effectors cocultured in vitro come from different donors, thereby adding the potential confounding factor of allogeneic reactions. Thus, we viewed these in vitro studies exploring the impact of T-cell help on RTX-mediated NK-cell ADCC as hypothesis generating. Here, we evaluated the role of T-cell help in RTX-mediated ADCC through in vivo analysis using both an animal model and clinical correlative analyses. The results provide further evidence in support of the hypothesis that T-cell help, provided in large part by intratumoral CD4+ T cells, contributes to the sustained activation of RTX-activated NK cells and their ability to mediate ADCC.
As is the case with in vitro studies, investigation of mechanistic questions with humanized mouse models and clinical correlative analyses each have both advantages and limitations. The humanized mouse model used for these studies allowed us to control the numbers of T cells and NK cells in the TME. This is a significant advantage over in vitro studies that it allowed for us to assess the changes in response to therapy in the actual TME. On the contrary, intratumoral immune infiltrates from these studies were generated from normal donor PBMCs, which are an imperfect surrogate for tumor-infiltrating lymphocytes. As with in vitro studies, there is a potential for an allogeneic response between the PBMCs and Raji cells. Such a model is also limited with respect to assessing the impact of myeloid cells on therapy because tumors contained few myeloid cells. Thus, this model served as only 1 approach to assess the hypothesis. Instead of evaluating the importance of T-cell help in another mouse model, we opted to assess it in clinical samples, the gold standard approach for assessing how therapy affects the TME. Practical considerations related to current standards of clinical care limited our ability to assess the impact of single-agent RTX on the TME, because few patients currently receive single-agent RTX. We performed serial FNAs on 10 patients who were receiving a more commonly used regimen for follicular lymphoma, namely bendamustine plus RTX. This was done with the understanding that bendamustine can suppress T-cell responses,28 which could potentially reduce T-cell help. Our identification of a correlation between T-cell help and NK-cell activation after RTX, despite the potential immunosuppressive effects of bendamustine, further supports the underlying hypothesis. Because bendamustine plus RTX is a highly effective regimen, it was not possible in a study of this size to determine a correlation between the numbers of CD4+ T cells in the pretherapy TME and the clinical outcome to therapy. Indeed, all but 1 patient in our study achieved a complete response to bendamustine- RTX therapy.
Despite the individual limitations of in vitro analysis reported previously and the animal model and clinical correlative results reported here, results from all 3 approaches are consistent. Conclusions reached from each approach are summarized in Table 2 and together provide strong evidence that T-cell help in the TME enhances RTX-mediated NK-cell ADCC. These results are consistent with previous reports of T cells, mainly CD4+ T cells being the primary producers of IL-2,29 which is known to induce NK-cell proliferation and cytotoxicity.30-35 Systemic therapy with IL-2 or IL-2–containing fusion proteins is known to enhance NK-cell–mediated ADCC,36,37 so it is not surprising that locally produced IL-2 has similar effects. Additionally, it is reported that CD16 engagement by RTX enhances CD25 on NK cells, resulting in increased sensitivity to IL-2.23 Further reinforcing this connection, we observed an increase in T-cell–dependent CD25 expression on NK cells, which suggests a link between locally produced IL-2 and enhanced NK-cell viability after NK cells are activated with RTX. This is consistent with our prior in vitro studies that showed T-cell help is particularly important for NK cells that are activated via CD16.22 Given the importance of NK-cell CD16 for RTX-mediated ADCC,6,10,14 it is reasonable to infer that patients with low numbers of CD4+ T cells in the TME will be less likely to respond to therapy, a result that is consistent with prior studies demonstrating a correlation between outcome in B-cell malignancies and a T-cell infiltrate.19-21 More extensive in vivo exploration of the role of IL-2 and other T-cell cytokines produced locally in the TME and their role in enhancing ADCC is ongoing.
Summary of conclusions based on in vitro, animal model and clinical correlative analysis
| . | In vitro analysis . | Animal model results . | Clinical correlative results . |
|---|---|---|---|
| T cells in the TME contribute to RTX-induced NK-cell viability and activation | ✓ | ✓ | ✓ |
| T-cell help enhances RTX-mediated target cell lysis | ✓ | ✓ | |
| T-cell help enhances the antitumor activity of RTX | ✓ |
| . | In vitro analysis . | Animal model results . | Clinical correlative results . |
|---|---|---|---|
| T cells in the TME contribute to RTX-induced NK-cell viability and activation | ✓ | ✓ | ✓ |
| T-cell help enhances RTX-mediated target cell lysis | ✓ | ✓ | |
| T-cell help enhances the antitumor activity of RTX | ✓ |
The potential clinical implications of this study are significant. It provides a basis for designing regimens capable of overcoming resistance due to low intratumoral T cells. Anti-CD3 bispecific antibodies (bsAbs) are showing great promise as treatments for B-cell malignancies.38 They activate T cells by bypassing the need for T-cell receptors to bind a peptide-MHC complex. The intended primary goal of treatment with bsAbs is to redirect cytotoxic T cells toward tumor cells, however, activation of T cells by bsAbs also induces cytokine production from T cells, including IL-2.7,39 These cytokines, particularly when produced locally, might then play a key role in enhancing T-cell help that supports NK-cell ADCC even when there are lower numbers of CD4+ T cells in the TME. Indeed, previous in vitro studies in our laboratory demonstrated that activating a low number of T cells using bsAbs enhances NK-cell numbers, CD16 expression, and ADCC mediated by RTX.40 Animal model and clinical correlative studies similar to those described here will begin shortly to assess the impact of bsAbs on T-cell help to enhance ADCC when low numbers of intratumoral T cells are present. Evaluation of real-world data sets, particularly gene expression data are being used to assess this hypothesis. Gene expression signatures can be deconvoluted to identify the immune infiltrates in the TME and correlated with therapeutic outcomes.41,42 We are also exploring whether similar mechanisms hold true with other mAbs and malignancies.
In conclusion, the data presented here using a novel mouse model and correlative results from clinical samples provide strong evidence that T-cell help in the TME enhances RTX-mediated NK-cell viability and ADCC in vivo. This provides new insight into an additional potential mechanism of resistance to therapy and to the design of new regimens that could allow us to make even better use of what are already well established and invaluable components of our therapeutic armamentarium.
Acknowledgments
The authors thank Suresh Veeramani, Sue E. Blackwell, and Travis D. Fischer for helpful discussions; Charles River for providing mice for these studies; and Flow Cytometry Core Facility, Small Animal Imaging Core Facility, and Comparative Pathology Laboratory at the University of Iowa.
This work was supported in part by National Institutes of Health, National Cancer Institute grants P50 CA097274 and P30 CA86862, and by a V Foundation Translational Research grant.
J.A. is a PhD candidate at University of Iowa. This work is submitted in partial fulfillment of the requirement for the PhD.
All illustrations were created with BioRender.com.
Authorship
Contribution: J.A. performed mice experiments, analyzed data, made figures, and wrote the manuscript; C.D.L.-M. performed mice experiments; S.A. and U.F. led correlative clinical trial; C.Y. and Z.W. performed flow cytometry; C.Y., J.A., Z.W., and G.J.W. analyzed clinical samples data; J.A. and B.J.S. performed statistical analysis; and G.J.W. provided oversight of the research project, designed the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: George J. Weiner, University of Iowa, Holden Comprehensive Cancer Center, 5210 MERF, 375 Newton Rd, Iowa City, IA 52242; email: george-weiner@uiowa.edu.
References
Author notes
Data are available on request from the corresponding author, George Weiner (george-weiner@uiowa.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal