Bourgeois and colleagues have identified that dual targeting of 2 transcriptional regulators, Ikaros and menin, is highly effective in distinct genetic subtypes of acute myeloid leukemia (AML).1 Despite the introduction of new therapies like inhibitors of BCL2, FLT3, and IDH1/2, the outcome in AML is still poor, and only ∼50% of intensively treated patients can be cured with current treatments. For elderly patients not eligible for intensive chemotherapy, the combination of a hypomethylating agent with the BCL2 inhibitor venetoclax has improved outcome, yet most patients relapse and, in the absence of effective second-line therapies, die from leukemia. Therefore, new treatment modalities with high leukemia selectivity are needed to improve survival in AML. Accordingly, the work by Bourgeois and colleagues builds on a study previously published by the group,2 where they showed that AML with lysine methyltransferase 2A (KMT2A, MLL1) rearrangements (KMT2Ar) depend on the zinc finger transcription factor Ikaros (IKZF1).
Ikaros is an important transcriptional regulator of lymphopoiesis and essential for many B-cell malignancies, but its role in myeloid malignancies is less clear. Thalidomide analogs, immunomodulatory imide drugs (IMiDs) like lenalidomide and pomalidomide, induce degradation of Ikaros and its homolog Aiolos via the cereblon E3 ubiquitin ligase leading to death of multiple myeloma cells and activation of T cells.3 In contrast to multiple myeloma, lenalidomide has only modest antileukemic activity on AML cells in vitro, and clinical trials with lenalidomide in unselected patients with AML provided mixed results.
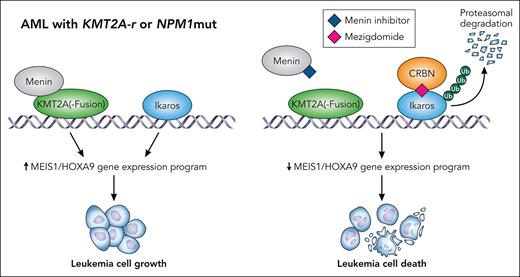
In the current work, the Armstrong group has now conducted compelling in vitro and in vivo studies using the newer Ikaros degrader mezigdomide, which is ∼1000 times more potent than lenalidomide.4 Although mezigdomide is currently in clinical trials for multiple myeloma and has activity in lenalidomide-resistant patients,5 Bourgeois and colleagues now demonstrate that it is also more active than lenalidomide in KMT2A-r AML cell lines and primary AML patient cells in vitro. Furthermore, mezigdomide can delay leukemia growth in AML patient-derived xenograft (PDX) models from both KMT2A-r and nucleophosmin (NPM1) mutant AML. In contrast, in most other AML genetic subtypes, mezigdomide did not have meaningful activity. These data clearly demonstrate that some AML subtypes are dependent on Ikaros, but more complete degradation seems to be needed in comparison with multiple myeloma. This can be achieved with novel cereblon modulators, which via Ikaros degradation suppress a HOXA9-associated gene expression program. This is induced by KMT2A rearrangements as well as NPM1 mutations and is essential for these AML subtypes, thus explaining their high sensitivity to mezigdomide.
Menin, which is encoded by the gene MEN1, was discovered as another important cofactor for KMT2A-r-mediated as well as NPM1 mutation–mediated transcriptional regulation of leukemia-associated gene expression programs including MEIS1 and HOXA9.6,7 Selective menin inhibitors have been developed that can induce remission in about 30% of patients with relapsed or refractory AML with NPM1 mutations even as monotherapy.8 However, about 40% of patients treated acquire mutations in MEN1 in the inhibitor binding domain, impairing activity of the inhibitor and leading to AML relapse.9
In the study by Bourgeois and colleagues, the combination of mezigdomide and the menin inhibitor VT-504690 was highly synergistic in vitro and was able to induce long-term remission in vivo in NPM1 mutated and KMT2Ar AML PDX mouse models. (see figure) Remarkably, mezigdomide prevented the development of MEN1 resistance mutations in vivo. Surprisingly, mezigdomide even sensitized AML cells with MEN1 resistance mutations to menin inhibitors, the mechanism of which is not entirely understood yet.
Combined targeting of Ikaros and menin has synergistic effects on AML with NPM1 mutations or KMT2A rearrrangement through inhibiting a MEIS1/HOXA9-associated gene expression program. Professional illustration by Patrick Lane, ScEYEnce Studios.
Combined targeting of Ikaros and menin has synergistic effects on AML with NPM1 mutations or KMT2A rearrrangement through inhibiting a MEIS1/HOXA9-associated gene expression program. Professional illustration by Patrick Lane, ScEYEnce Studios.
In summary, the study revealed a new potent combination therapy targeting leukemia-associated transcriptional networks. The comprehensive preclinical experiments provide a great basis for quick translation of this new therapeutic approach into clinical trials for 2 genetically defined AML subtypes that account for ∼40% of all cases. Although currently neither menin inhibitors nor Ikaros degraders are used as standard therapy in AML, the dual menin and Ikaros inhibition is a promising approach in patients relapsing after chemotherapy or venetoclax/hypomethylating agent combination therapy. Importantly, the combination of mezigdomide and menin inhibition had only modest toxicity on healthy human cells in vitro and hematopoiesis in IMiD-sensitive mouse models, thereby providing a therapeutic window for AML. This would potentially also allow to add this combination to the standard venetoclax and hypomethylating agent first-line therapy. Ultimately, this could then finally bring also NPM1-mutated and KMT2Ar AML so close to the sun that it will melt, like the wax wings of Ikaros, the son of Daedalus, when he was flying too high.
Conflict-of-interest disclosure: L.B. declares honoraria: AbbVie, Amgen, Astellas, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Janssen, Jazz Pharmaceuticals, Novartis, Pfizer, Sanofi; consulting or advisory role: AbbVie, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Gilead, Hexal, Janssen, Jazz Pharmaceuticals, Menarini, Novartis, Pfizer; speakers bureau: AbbVie, Amgen, Astellas, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Janssen, Jazz Pharmaceuticals, Novartis, Pfizer, Sanofi; Research. J.K. declares consulting, advisory board, speakers bureau, honoraria, travel support: Bristol Myers Squibb, Celgene, Takeda, Janssen, AbbVie, Sanofi, Pfizer.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal