In this issue of Blood, Vadivel et al showed that Staphylococcus aureus promotes therapeutic resistance to several drugs commonly used in the treatment of patients with epidermotropic cutaneous T-cell lymphomas (CTCLs) that include mycosis fungoides and Sézary syndrome.1
S aureus is a commensal Gram-positive commensal bacterium, which is part of the normal skin microbiome. Since the report of toxin-producing S aureus colonization of lesional skin of patients at advanced CTCL stage,2 numerous studies have provided evidence that S aureus plays a role in CTCL pathogenesis. S aureus toxins, such as staphylococcal enterotoxins (SEs) and staphylococcal alphatoxin, act on malignant T cells either directly through superantigen stimulation or indirectly through the release by the tumor microenvironment of growth factors, cytokines, and oncogenic microRNAs.3 In addition, a recombinant bacteriophage-produced endolysin (XZ.700) was recently used to inhibit both the effects of S aureus on activation and proliferation of malignant T cells and cell lines in the presence of nonmalignant T cells.4
Vadivel et al identified that SE can induce resistance of Sézary cells to histone deacetylase inhibitors, such as romidepsin, vorinostat, and reminostat.1 SE obtained for supernatants from lesional skin of patients with CTCL or a recombinant SE blocked romidepsin-induced apoptosis of Sézary peripheral blood cells. The protective effect was not observed with supernatants of endolysin-treated SE-producing S aureus or with supernatants from S aureus that did not produce SE.
SE protective effect was also observed with drugs inducing DNA damage, such as doxorubicin and etoposide, as well as with the adenosine triphosphate synthase inhibitor oligomycin.1 SE did not induce drug efflux but activated specific pathways. Single-cell indexing of transcriptomes and epitopes by sequencing (CITE-seq) of Sézary cells, treated by romidepsin in the presence or absence of SE, identified induction of NF-κB and T-cell receptor (TCR)/B-cell receptor signaling on SE exposure. The NF-κB inhibitor bortezomib abrogated the SE-induced drug resistance. Inhibition of TCR signaling by protein tyrosine kinase–like inhibitors or the protein kinase C-θ inhibitor sotrastaurin abrogated NF-κB activation and the SE-protective effect. Highly purified staphylococcal enterotoxin A (SEA; the major type of SE in this study) provided the protective effect. It was abolished by mutations in the major histocompatibility complex (MHC) class II binding domain that prevent further TCR activation of a recombinant SEA or by an anti-SEA blocking antibody. Altogether, these data supported a direct protective effect of SE for malignant T cells.
CITE-seq data indicated that SE stimulation also induced cytokine-mediated JAK/STAT signaling in both malignant and nonmalignant T cells either directly or through the release of interleukin-2 (IL-2) family cytokines. Using A-419259 to block TCR signaling, they observed that SE-induced romidepsin resistance was abrogated by TCR-signaling inhibition but that the protective effect of IL-2 family cytokines was only blocked by the clinically used JAK inhibitor, tofacitinib. This dual effect was clinically relevant as the JAK inhibitor had little or no impact on the SE-protective effect on peripheral blood cells in 3 of 5 patients as opposed to inhibition observed in the 2 other patients’ samples. Heterogeneity of Sézary cells is already known and was confirmed by patients’ derived cell lines.5 SE-direct effect on Sézary cells may therefore depend on their maturation stage, MHC II binding, and TCR-signaling properties.
In parallel to the present report,1 recent findings underscored the pathogenic role of S aureus in the skin. In a large cohort of Asian patients with CTCL, microbiome disequilibrium with predominance of S aureus was present in the lesional skin of patients with advanced stage disease.6 These authors identified the same S aureus strain between lesional and nonlesional skin, according to culture results and enterotoxin type. Staphylococcal enterotoxin B (SEB) was predominant (18.2%) over SEA (9.1%) and staphylococcal enterotoxin C (SEC) (4.5%).6 Whether SEB and SEC have similar effects to SEA on CTCL cells needs further evaluation. These data point to the need for skin bacterial culture to evaluate therapeutic resistance in CTCL clinical trials. As S aureus colonization is a universal feature of CTCL worldwide, these studies support the use of combination therapy with antibiotics, especially for patients with refractory or advanced CTCL.1,3,6 Preventing lesional skin colonization at early CTCL stages by long-term aggressive antibiotic treatment does not appear advisable because of the life-threatening risk of selecting antibiotic-resistant strains. Nonantibiotic tools, such as the engineered endolysin XZ.700, need further evaluation in combination therapy as it prevented ex vivo colonization by S aureus of both healthy and lesional skin.4
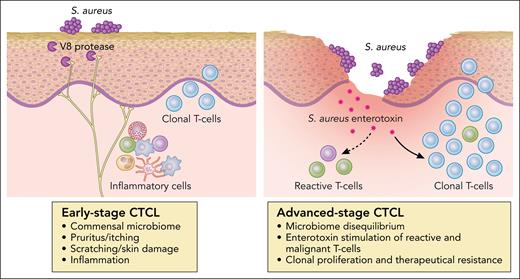
Fighting S aureus may also considerably improve quality of life of patients with CTCL. A major role of S aureus in skin diseases was recently elucidated through the identification of the link between S aureus and pruritus or itch.7 The causative role for microbes in driving itch was previously unknown, although many skin diseases, such as atopic dermatitis (AD) or impetigo, characterized by itchy lesions were associated with S aureus colonization and subsequent inflammation.8 Severe pruritus is also a major distressing symptom in patients with CTCL, especially in Sézary syndrome. Pruritus in CTCL is long lasting and refractory to standard treatment, leading to the use of cytokine receptor inhibitors, such as dupilumab, that may have a counterproductive effect on CTCL exacerbation.9 Using a mouse AD model, Deng et al tested multiple isogenic S aureus mutants for virulence factors and identified the S aureus serine protease V8 as a critical factor.7 V8 cleaves the proteinase-activated receptor 1 (PAR1) on mouse and human sensory neurons (see figure). Inhibition of the PAR1 activation was found to block and prevent itch, which was not found to require preexisting skin inflammation or disruption.7 In patients with CTCL, S aureus may induce pruritus and subsequent scratch skin-barrier damage favoring generalized infections, a major cause of death.10 Inhibition of pruritus and prevention of S aureus colonization could also hamper progression of early-stage CTCL.
The deleterious effects of S aureus in CTCLs. Professional illustration by Patrick Lane, ScEYEnce Studios.
The deleterious effects of S aureus in CTCLs. Professional illustration by Patrick Lane, ScEYEnce Studios.
In summary, S aureus appears to be a promoter of CTCL progression and therapeutic resistance. Targeting its deleterious effects is mandatory for patients’ outcome as well as for their quality of life.
Conflict-of-interest disclosure: J.-P.M. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal