In this issue of Blood, Namazzi and colleagues1 report a secondary analysis of a trial of zinc2 in 248 Ugandan children with sickle cell anemia showing that the 117 children who were started on hydroxyurea as part of their routine sickle cell anemia care during the course of the study had a significant reduction in malaria and other infections (see figure). The parent study of all 248 children did not demonstrate a reduction in infection with zinc compared with placebo.
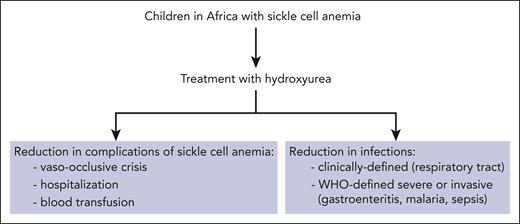
When children in Africa with sickle cell anemia receive hydroxyurea, evidence points to reductions in both complications of sickle cell anemia and risk of infections.
When children in Africa with sickle cell anemia receive hydroxyurea, evidence points to reductions in both complications of sickle cell anemia and risk of infections.
Malaria and other infections are important health problems and major causes of death among children in Africa, including those with sickle cell anemia.3 Hydroxyurea in the doses used to treat sickle cell anemia tends to reduce the neutrophil count, occasionally to a profound extent, and one of the concerns with its use in the African setting is that it may increase the risk of infection.4 However, studies by the group of which Ruth Namazzi is a part have provided evidence that hydroxyurea can be used safely in African children with sickle cell anemia without an increased risk of infection.5,6
When Namazzi and colleagues started the trial of zinc, almost none of the children with sickle cell anemia randomized to zinc or placebo were receiving hydroxyurea. Based on increasing evidence of the safety and efficacy of hydroxyurea in children in Africa7 and on guidelines of the Ugandan Ministry of Health, the investigators recommended treatment with hydroxyurea for all study participants and provided the agent free of charge. Most parents were initially hesitant to agree to hydroxyurea, but by the end of the study hydroxyurea was prescribed for about half (117 of 248) of the children. The average duration of hydroxyurea therapy was 7.5 months.
The incidence of infections can be seasonal, which could influence the results of a secondary analysis like the present report. The investigators carefully considered this possibility in their analysis and ruled out seasonality as the cause of the decrease in infections seen after hydroxyurea was added to the zinc or placebo.
The investigators rigorously categorized all infections occurring during the trial using 2 prospectively defined criteria: (1) severe or invasive infections based on World Health Organization (WHO) criteria (such as malaria, sepsis, bacteremia, gastroenteritis, and sinusitis) and (2) infections clinically defined according to the study protocol (such as lower respiratory tract and upper respiratory tract infections). In both cases, hydroxyurea treatment was associated with highly significant reductions in infections. Examples of infections that were reduced during hydroxyurea treatment include malaria and gastroenteritis in category 1 and lower respiratory tract infections in category 2. The mechanism(s) by which hydroxyurea protects against infection are not certain, but possibilities include improved immune function, preserved splenic function, anti-inflammatory properties, and direct antimicrobial activity.8,9
Limitations of the report are that it is based on a secondary analysis of a randomized trial of another agent and that compliance with hydroxyurea was not monitored with pill counts. Therefore, further studies are needed to confirm the reported reduction in infections in children with sickle cell anemia who receive hydroxyurea and to determine the mechanism(s) involved in this beneficial effect.
Nevertheless, the findings that hydroxyurea treatment in children with sickle cell anemia in Africa not only reduces complications of the underlying condition but also appears to reduce clinically defined infections and WHO-defined severe infections are indeed glad tidings that should bring great joy to these children and their families.
Conflict-of-interest disclosure: V.R.G. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal