In this issue of Blood, Han et al1 show that erythropoiesis is impaired in a sickle cell disease mouse model because of hemolysis-induced inflammation and interferon-α production that promotes erythroid CISH (cytokine-inducible SH2 [Src homology 2 domain]-containing protein) expression, a negative regulator of erythropoietin signaling. Although hemin treatment suppresses erythropoiesis, knockout of interferon-α receptor 1 improves the defective bone marrow erythropoiesis in sickle cell mice. The study by Han et al identifies an unexpected role for the heme–interferon-α–CISH axis in impaired erythropoiesis in sickle cell disease (see figure) that may be relevant for erythropoietin resistance and anemia of chronic disease.
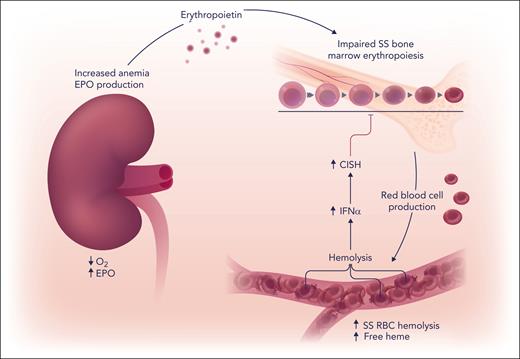
Anemia in sickle cell disease increases hypoxia induction of erythropoietin (EPO) in the kidney to stimulate bone marrow erythropoiesis and red blood cell (RBC) production. In the circulation, polymerization of deoxygenated sickle hemoglobin leads to repeated sickle (SS) erythrocyte sickling and unsickling, increased red blood cell fragility, and oxidative stress that causes intravascular hemolysis. The release of cell-free heme in the circulation promotes a proinflammatory state and increases interferon-α (IFN-α) that increases erythroid expression of suppressor of cytokine signaling family member, CISH. CISH decreases erythropoietin signaling in erythroid cells, resulting in impaired bone marrow erythropoiesis. Professional illustration by Somersault18:24.
Anemia in sickle cell disease increases hypoxia induction of erythropoietin (EPO) in the kidney to stimulate bone marrow erythropoiesis and red blood cell (RBC) production. In the circulation, polymerization of deoxygenated sickle hemoglobin leads to repeated sickle (SS) erythrocyte sickling and unsickling, increased red blood cell fragility, and oxidative stress that causes intravascular hemolysis. The release of cell-free heme in the circulation promotes a proinflammatory state and increases interferon-α (IFN-α) that increases erythroid expression of suppressor of cytokine signaling family member, CISH. CISH decreases erythropoietin signaling in erythroid cells, resulting in impaired bone marrow erythropoiesis. Professional illustration by Somersault18:24.
Erythropoietin regulates bone marrow erythropoiesis for production of red blood cells that are responsible for oxygen transport from the lungs to the tissues. Mouse models for β-hemoglobinopathies, such as β-thalassemia2 and sickle cell disease,3 provide insight into the complex pathophysiology associated with the resultant anemia and erythropoietin-driven erythropoietic response. Causes of anemia include ineffective erythropoiesis and hemolysis. Ineffective erythropoiesis in β-thalassemia is associated with the imbalance of erythropoietin-stimulated expansion of early-stage erythroid progenitor cells and disrupted differentiation of late-stage erythroid precursors to circulating red blood cells.4 Peripheral hemolysis in sickle cell anemia is largely a consequence of polymerization of deoxygenated sickle hemoglobin causing repeated sickle (SS) erythrocyte sickling and unsickling, increased erythrocyte fragility, and oxidative stress.5,6 Associated intravascular hemolysis in sickle cell disease releases cell-free heme that contributes to decreased nitric oxide bioavailability and promotes a proinflammatory state, increasing inflammatory cytokines, such as interleukin-6, placental growth factor, and endothelin-1, and disease-associated vaso-occlusion, pulmonary hypertension, and organ damage.5
Endogenous erythropoietin was elevated by about ≥5-fold in both anemic β-thalassemia and sickle cell (SS) mouse models. Although β-thalassemia mice exhibited 2- to 3-fold increased bone marrow erythropoietic activity with increased erythroblasts and erythroid colony-forming ability, in SS mice, bone marrow erythropoietic activity was impaired and only modestly increased compared with AA control mice expressing normal adult human hemoglobin. Sorted SS erythroid progenitor cells showed decrease erythroid colony-forming ability and a decreased response to erythropoietin compared with AA control. Hemolysis in sickle cell disease increases cell-free heme and hemin injection into AA mice, used to model chronic intravascular hemolysis, resulted in reduced bone marrow erythropoiesis and erythroid progenitor cell response. Similarly, injection of red cell lysate into wild-type mice decreased bone marrow erythroid colony formation and erythroblast number, and erythropoietin stimulated Stat5 and extracellular signal-regulated kinase signaling in erythroid cells. However, hemin treatment did not affect cultures of erythropoietin-stimulated bone marrow erythroid cells or Stat5 phosphorylation, indicating that hemolysis and/or hemin-induced suppression of erythropoiesis in vivo in mice is via an indirect mechanism.
Increased hemolysis is associated with increased interferon-α, and plasma heme levels were shown to correlate with circulating interferon-α in patients with sickle cell disease.7 Interferon-α levels were elevated in SS mice7 and in AA mice with heme injection. Interferon-α treatment in AA mice decreased bone marrow erythropoiesis, increased erythroid cell expression of suppressor of cytokine signaling family member, Cish, and decreased erythropoietin response in erythroid progenitor cell cultures. Cish expression was also elevated in bone marrow erythroid cells from SS mice with increased endogenous interferon-α. Knockout of interferon-α receptor 1 attenuated the reduction in mouse bone marrow erythropoiesis with hemin treatment and increased bone marrow erythropoietic activity and erythropoietin-stimulated erythroid cell response and decreased erythroid cell Cish expression in SS mice. Deletion of interferon-α receptor 1 rescued the impaired bone marrow erythropoiesis in SS mice without changing peripheral blood red blood cell count, hemoglobin, hematocrit, and reticulocyte number. Analogous to mouse erythroid cell response, human erythroid cultures treated with interferon-α activated interferon signaling, inhibited cell growth and erythropoietin-stimulated STAT5 phosphorylation, and induced CISH expression.
This research provides a mechanistic understanding for the impaired erythropoietic response in sickle cell disease to endogenous erythropoietin and relates to the higher erythropoietin dose required for treatment of anemia in sickle cell disease compared with patients with chronic kidney disease. The heme–interferon-α–CISH axis is identified as a contributing factor to impaired erythropoietin-stimulated erythropoietic response in SS mice that may also relate to anemia of chronic disease and erythropoietin resistance, but will require further validation in humans. These results are especially timely with the increasing availability of hypoxia-inducible factor–proline hydroxylase inhibitors that increase production of endogenous erythropoietin and suggest the potential necessity to increase dosage for treatment of anemia in patients with sickle cell disease.
Deletion of interferon-α receptor 1 partially rescued the hemolysis-associated impaired erythropoiesis, providing evidence for the important role of heme–interferon-α–CISH axis and suggesting contributions from interferon-α–independent mechanisms. Other processes that may affect erythropoietin-dependent erythropoiesis in response to hemolysis and increased free heme that warrant further investigation include induction of other inflammatory cytokines, such as interleukin-6, macrophage function to form erythroid blood islands, and marrow mesenchymal stromal cell formation of the hematopoietic stem cell niche to support erythropoiesis. Decreased nitric oxide bioavailability may also decrease erythropoiesis, as suggested by the decreased erythropoietic response to erythropoietin treatment in mice that lack neuronal nitric oxide synthase.8
Han et al identified the heme–interferon-α–CISH axis as a significant mediator of impaired erythropoiesis in a mouse model of sickle cell disease, despite the marked increase in endogenous erythropoietin production, which could account for the higher erythropoietin dose necessary to treat anemia in patients with sickle cell disease. Beyond therapeutic strategies that focus on hemolysis and/or deoxygenated sickle hemoglobin polymerization, alternative approaches are suggested, such as targeting heme-induced inflammatory response, including interferon signaling or induction of CISH. Such approaches may also extend to erythropoietin resistance in other disease states, including anemia of chronic disease.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal