In this issue of Blood, Chagraoui et al1 uncover new insights into the mechanism of action of the small molecule agonist of ex vivo human hematopoietic stem cell (HSC) expansion, UM171.
HSCs are a rare multipotent self-renewing stem cell population with the ability to regenerate the entire blood and immune systems following transplantation into a myeloablated recipient. Allogeneic HSC transplantation is a key therapy in the treatment of various serious blood diseases including acute myeloid leukemia.2 Most patients receiving transplants get HSCs provided by a healthy human leukocyte antigen (HLA)-matched adult donor. HLA matching is important to decrease the risk of graft-versus-host disease (GvHD), which can have serious toxicities. Unfortunately, finding a suitably matched healthy donor can be challenging, particularly for patients from minority populations.
Umbilical cord blood provides a rich source of HSCs that can be readily collected, banked, and used for HSC transplantation.2 Use of cord blood also reduces the risk of GvHD. However, a single unit of cord blood often contains too few HSCs to efficiently engraft, which limits the number of available units (and HLA representation). The clinical risks are also increased. In particular, patients with transplants rapidly become neutropenic following myeloablative conditioning, which makes them highly susceptible to opportunistic infections that can be fatal. Neutrophil reconstitution is slower from cord blood, often taking 3 weeks, resulting in an extended period of vulnerability for the patient.
Given the accessibility yet paucity of cord blood HSCs, ex vivo expansion and transplantation of higher HSC doses has been proposed as a strategy to improve the safety and availability of cord blood transplantation.3 Large research efforts have therefore focused on developing approaches to expand transplantable HSCs ex vivo. However, HSCs rapidly lose transplantation capacity when placed into standard ex vivo culture conditions containing hematopoietic growth–promoting cytokines. Efficient HSC expansion ex vivo has therefore remained a major challenge in the field for many years.3
In 2014, the Sauvageau laboratory discovered one of the first small molecule agonists of ex vivo expansion of umbilical cord blood HSCs, the pyrimidoindole-derivative UM171, which supported ∼30-fold expansion of transplantable HSCs in 10-day cultures.4 This discovery led to a phase 1/2 clinical trial in 2016 that tested UM171-expanded cord blood in allogeneic HSC transplantation for patients with hematological malignancies.5 The trial demonstrated safety and feasibility of the approach and improved engraftment parameters. In particular, the UM171-expanded product reduced neutrophil reconstitution time by several days.5
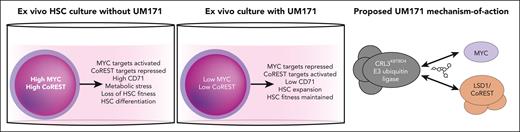
Despite its clinical development, the mechanism of action of UM171 remained completely unknown until 2020 when it was shown to mediate degradation of lysine-specific histone demethylase 1A (LSD1 or KDM1A) and its associated CoREST epigenetic complex.6,7 At the molecular level, UM171 essentially acts as a proteolysis targeting chimera molecule by recruiting the E3 ubiquitin ligase CRL3KBTBD4 complex, which results in target protein ubiquitination and proteasomal degradation.7 However, LSD1 inhibitors failed to completely recapitulate the UM171 phenotype, suggesting that this target did not fully explain the mechanism of action.
In this new study, Chagraoui et al now solve another piece of the UM171 puzzle by identifying MYC as another key UM171 target (see figure). MYC is an important transcription factor that acts as a key node regulating the cell cycle, transcription, translation, and metabolism (including autophagy).8 Chagraoui et al noticed MYC target genes were significantly repressed in UM171-treated cells. They followed up on this observation by confirming a loss of MYC protein following UM171 treatment. Consistent with the known roles of MYC, UM171 also reduced rates of translation and increased lysosome content. Additionally, expression of a degradation-resistant version of MYC reversed the HSC expansion mediated by UM171. Finally, this study also suggested that low expression of the MYC-target CD71 can help to enrich for functional UM171-expanded HSCs.
Multiple mechanisms of UM171 promote ex vivo HSC expansion. In standard human HSC culture conditions, high levels of MYC and CoREST accumulate, resulting in loss of transplantable HSCs. UM171 induces human HSC expansion ex vivo, at least in part, by degrading MYC and the LSD1/CoREST complex via its ability to recruit them to the CRL3KBTBD4 E3 ubiquitin ligase.
Multiple mechanisms of UM171 promote ex vivo HSC expansion. In standard human HSC culture conditions, high levels of MYC and CoREST accumulate, resulting in loss of transplantable HSCs. UM171 induces human HSC expansion ex vivo, at least in part, by degrading MYC and the LSD1/CoREST complex via its ability to recruit them to the CRL3KBTBD4 E3 ubiquitin ligase.
Why is this important? First, this study provides novel mechanistic insights into what is required for HSCs to expand ex vivo. These results give clues to why human HSCs struggle to grow (without UM171) ex vivo but not in vivo. Induction of high levels of MYC in ex vivo culture (likely driven by the high cytokine and nutrient availability) appear to induce cellular stresses that drive loss of HSC fitness and functional capacity. These new insights might also help to explain why other targeted small molecule approaches have struggled to efficiently boost ex vivo HSC expansion. UM171 targets multiple distinct pathways simultaneously; degradation of at least LSD1/CoREST and MYC seem required for the full effects of UM171.
Second, UM171 remains one of the most potent small molecule agonists of ex vivo human HSC expansion for HSC research. While cultures containing recombinant cytokines and UM171 support expansion of transplantable HSCs for up to 2 weeks, a new chemically defined culture condition was recently developed that could support expansion of transplantable human HSCs for over 4 weeks.9 This longer-term culture protocol opens the door for deeper biological investigations, ex vivo disease modeling, and next-generation HSC therapies. Notably, however, UM171 was also required to stabilize human HSCs in this chemically defined culture highlighting the importance of this molecule, and its mechanism of action, to the field.
More broadly, these studies highlight the potential for developing UM171 as a MYC degrader. MYC is commonly dysregulated in cancer, and many cancers rely on MYC to survive and proliferate.8 However, as a transcription factor MYC has been challenging to target therapeutically. These studies therefore suggest the potential to expand the use UM171 beyond HSCs.
Conflict-of-interest disclosure: The author serves as a consultant for ImmuneBridge Therapeutics.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal