Introduction: Despite governmental and industry-sponsored efforts, clinical trial participation among rural residents and racial and ethnic minorities remains low. The NIH Revitalization Act of 1993 directed the NIH to establish guidelines for inclusion of women and minorities in clinical research. In 2015, the Food and Drug Administration launched an action plan to improve the quality of demographic subgroup data collection, identify barriers to enrollment, employ strategies to increase participation, and promote transparency of related data. However subsequent studies (Green et al. Health Aff. March 2022, Kanapuru et al. Blood Advances. March 2022.) showed no improvement in participation of racial minorities, including Blacks and Hispanics since this plan was enacted. Various complex system- and individual-based factors, including lack of clinical trial availability; lack of diversity in the investigator work force; scant patient and provider awareness of clinical trials; lack of trust in the health care system; and issues with access to care-often driven by financial, geographic, or social constraints (Allison et al. Cureus. Apr 2022.), and stringent eligibility criteria are deemed responsible. The Leukemia & Lymphoma Society (LLS) IMPACT (Influential Medicine Providing Access to Clinical Trials) research grant strives to bridge this unrelenting care gap.
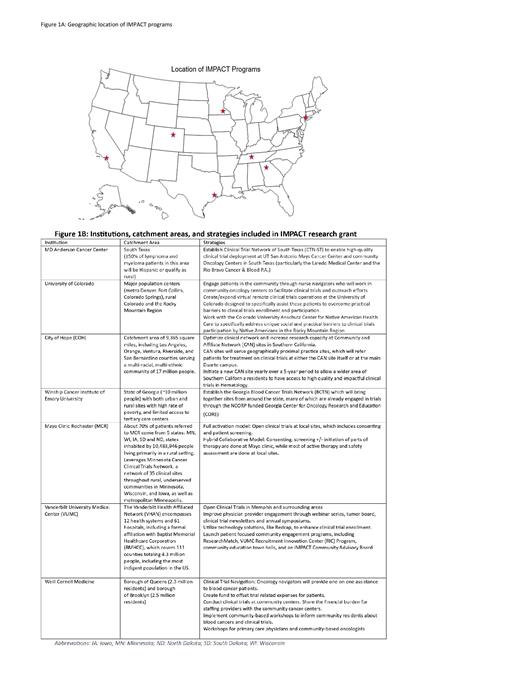
Methods: The LLS IMPACT program includes a 5-year research grant which was launched in 2021. The IMPACT grants support the establishment of clinical trial networks involving academic cancer centers (“Hubs”) linked to community clinics and oncology centers (“Spokes”). The program's overall goal is to provide patients access to trials through participation at the community sites. Initially, 3 institutional sites were selected to participate. These institutions had unique and tailored strategies to improve engagement of underrepresented patients, including black, indigenous, people of color, Hispanic, Latina/Latino, and people from rural communities in vastly different climates and local cultures. The IMPACT grant expanded to encompass 4 additional sites given the imminent needs and clamor of the hematology clinical trials community (Figure 1A). The catchment areas and strategies employed at each institution are summarized in the table (Figure 1B).
Results: At the start of the grant period, focus was on building clinical research infrastructure and opening non-interventional clinical trials to teach personnel about clinical research and create relationships with patients. Other programs such as webinar series, tumor boards, technological advancements, and educational workshops were also established to enhance engagement among physicians, match patients to clinical trials, and to provide clinical trial education to potential patients. One trial that has seen early success is the CHIVE IMPACT trial, a non-interventional registry and biorepository which has enrolled 24 patients at a rural and underserved site as of July 2023. The purpose of CHIVE is to enroll patients who have clonal hematopoiesis (CH) or are at risk of CH and follow prospectively in efforts to understand risk of progression and potential for early intervention. The Universal Protocol is another biorepository trial which has more than tripled its enrollment number at a community site between 2021 and 2022. Of the 63 patients enrolled in 2022, 66% of them identified as a race other than White.
As our core goal is to increase access to interventional trials, we hope to bring industry sponsored phase II and phase III studies to underrepresented areas that serve a broad patient population. Lack of financial resources, lack of transportation and distance to trial sites, and residual distrust of the medical community (e.g., legacy of Tuskegee experiments) are barriers the grantees are focused on overcoming to enroll the intended populations into clinical trials.
Conclusion: The innovative strategies employed to expand access to clinical trials for underrepresented population at the various institutions through the LLS's IMPACT research grant can be utilized during clinical trials design and implementation phases to improve generalizability of clinical trials in hematologic malignancies. In the third year of this program, 7 participating sites are engaging impactful strategies (Figure 1B) which will be reported at the end of the grant period.
Disclosures
Biltibo:BeiGene: Honoraria. Cohen:Loxo/Lilly: Consultancy, Research Funding; BeiGene: Consultancy; Janssen: Consultancy; Abbvie: Consultancy; AstraZeneca: Consultancy, Research Funding; ADCT: Consultancy; Takeda,: Research Funding; Novartis: Research Funding; BMS/Celgene: Research Funding; Genentech: Research Funding; BioInvent: Research Funding; Lam Therapeutics: Research Funding. Flowers:Abbvie: Consultancy, Research Funding; Bayer: Consultancy, Research Funding; Beigene: Consultancy; Celgene: Consultancy, Research Funding; Denovo Biopharma: Consultancy; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Genentech Roche: Consultancy, Research Funding; Genmab: Consultancy; Gilead: Consultancy, Research Funding; Karyopharm: Consultancy; N-Power Medicine: Consultancy, Current holder of stock options in a privately-held company; Pharmacyclics Jansen: Consultancy; SeaGen: Consultancy; Spectrum: Consultancy; 4D: Research Funding; Acerta: Research Funding; Adaptimmune: Research Funding; Allogene: Research Funding; Amgen: Research Funding; Cellectis: Research Funding; Guardant: Research Funding; Iovance: Research Funding; Jannsen Pharmaceuticals: Research Funding; Kite: Research Funding; Morphosys: Research Funding; Nektar: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Pharmacyclics: Research Funding; Sanofi: Research Funding; Takeda: Research Funding; TG Therapeutics: Research Funding; Xencor: Research Funding; Ziopharm: Research Funding; Burroghs Wellcome Fund: Research Funding; Eastern Cooperative Oncology Group: Research Funding; National Cancer Institute: Research Funding; V Foundation: Research Funding; Cancer Prevention and Research Institute of Texas: Research Funding; CPRIT Scholar in Cancer Research: Research Funding. Smith:OncoVerity: Current Employment, Current holder of stock options in a privately-held company; AML JV: Consultancy. Leonard:AbbVie, AstraZeneca, Astellas, Bayer, BeiGene, BMS, Calithera, Constellation, Eisai, Epizyme, GenMab, Grail, Incyte, Janssen, Karyopharm, Lilly, Merck, Mustang Bio, Pfizer, Roche/Genentech, Seagen, Second Genome, Sutro: Consultancy; National Cancer Institute, Leukemia and Lymphoma Society, Genentech, Epizyme, Janssen: Research Funding. Nowakowski:MEI Pharma: Consultancy; Seagen: Consultancy; Selvita Inc: Consultancy; Zai Lab Limited: Consultancy; Kymera Therapeutics: Consultancy; MorphoSys: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Consultancy; Abbvie: Consultancy; Curis: Consultancy; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Fate Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Ryvu Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy; Bantam Pharmaceutical LLC: Consultancy; Blueprint Medicines: Consultancy; Celgene Corporation: Consultancy; Debiopharm: Consultancy; F Hoffmann-La Roche Limited: Consultancy; Genentech: Consultancy; Incyte: Consultancy; Karyopharm Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Consultancy. Savona:AbbVie Inc.: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; CTI BioPharma Corp.: Membership on an entity's Board of Directors or advisory committees; Forma Therapeutics Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Geron Corporation: Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics Inc.: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Ryvu Therapeutics: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology, Inc.: Membership on an entity's Board of Directors or advisory committees; Taiho: Membership on an entity's Board of Directors or advisory committees; Takeda Pharmaceutical Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Boehringer Ingelheim: Patents & Royalties; ALX Oncology: Research Funding; Astex Pharmaceuticals: Research Funding; Incyte Corporation: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal