Introduction: Findings from a previously conducted matching-adjusted indirect comparison (MAIC) between isatuximab plus pomalidomide and dexamethasone (IsaPd) and daratumumab plus pomalidomide and dexamethasone (DaraPd) among relapsed and/or refractory multiple myeloma (RRMM) patients receiving at least two prior lines of therapy (LoT) including lenalidomide and a proteasome inhibitor (PI) demonstrated a significantly better overall survival (OS) and a positive trend for progression-free survival (PFS), overall response rate (ORR), and very good partial response or better (≥VGPR) rates with IsaPd than that with DaraPd, with a similar safety profile for both treatments [Leleu X et al, Value in Health 2022;25(1):S39]. The present study used longer follow-up data from the phase 3 ICARIA trial (NCT02990338) to update the previous MAIC, evaluating the comparative efficacy of IsaPd versus DaraPd.
Methods: A MAIC was performed with the latest available individual patient-level data (IPD) from the ICARIA trial (IsaPd arm, n = 154; Pd arm, n = 153), data cutoff of March 14, 2022, and aggregate data from study publications of the phase 3 APOLLO trial (DaraPd arm, n = 151; Pd arm, n = 153, NCT03180736) [Dimopoulos MA, et al. Blood 2022;140 (Suppl 1):7272-7274; Dimopoulos MA, et al. Lancet Oncol. 2021;22(6):801-812; Dimopoulos MA, et al. Blood 2020;136(Suppl 1):5-6]. To match patients with APOLLO, patients in ICARIA who did not meet the inclusion criteria of APOLLO were excluded. In APOLLO, 11% patients received only one prior LoT. As patients with one prior LoT were not enrolled in ICARIA, it was not feasible to match on this factor; however, the treatment effect of DaraPd versus Pd on PFS among these patients was similar to patients in APOLLO with ≥2 prior LoTs. Therefore, this difference in study populations is unlikely to bias the MAIC. Remaining patients in ICARIA were then weighted using standardized MAIC weights (mean value = 1.0) generated using the method of moments, with active treatment and control arms matched separately to ensure any residual imbalances between active and control arms in APOLLO were replicated in the matched ICARIA sample. Anchored MAICs for the hazard ratio (HR) for OS and PFS, odds ratio (OR) for ORR, and ≥VGPR for IsaPd versus DaraPd were calculated using the Bucher method [Bucher HC, et al. J Clin Epidemiol. 1997;50(6):683-691].
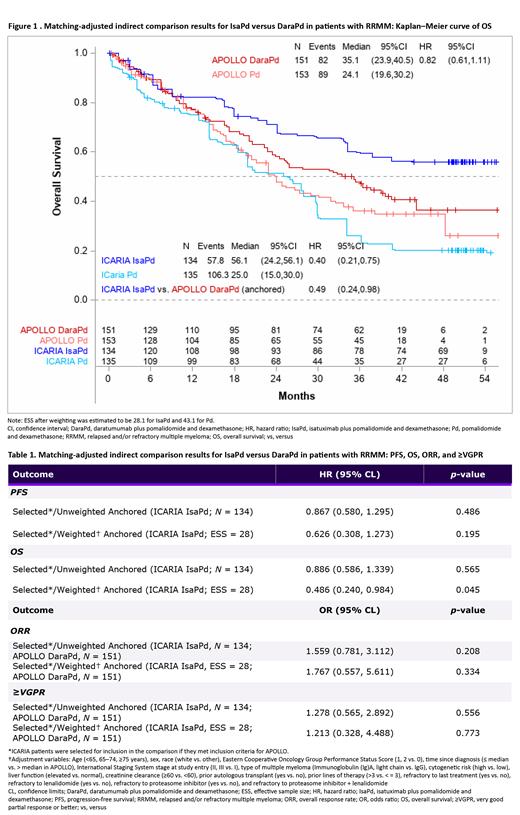
Results: MAIC weighting was able to match all characteristics included in the matching algorithm, with standardized mean difference (SMDs) <0.0001 after weighting. The effective sample size after weighting was 28.1 for IsaPd and 43.1 for Pd. Based on an anchored MAIC, OS was significantly better with IsaPd versus DaraPd (HR [95% CI]: 0.49 [0.24-0.98]; p = 0.04) (Figure 1). A numerical trend in favor of IsaPd versus DaraPd was observed for PFS, ORR, and ≥VGPR, although the differences were statistically non-significant (Table 1).
Conclusions: This MAIC analysis demonstrated a significant benefit in OS and a positive trend in the improvement of PFS, ORR, and ≥VGPR in favor of IsaPd compared with DaraPd. These findings were consistent with the previous MAIC with a longer follow-up data (present analysis used a maximum follow-up of 4.5 years of OS data from both trials, whereas the previous analysis used a follow-up of 3 years from ICARIA and unknown from APOLLO), which highlights the therapeutic potential of IsaPd in RRMM, particularly for patients who become refractory to lenalidomide and a PI.
Disclosures
Leleu:AbbVie: Honoraria; Amgen: Honoraria; Harpoon Therapeutics: Honoraria; GSK: Honoraria; Sanofi: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Janssen: Honoraria; BMS/Celgene: Honoraria; Merck: Honoraria. Delea:AbbVie: Research Funding; Amgen: Research Funding; Bristol-Myers Squibb: Research Funding; Celgene: Research Funding; Merck: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Regeneron: Research Funding; Sanofi: Research Funding; Takeda: Research Funding. Guyot:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Moynahan:Bristol-Myers Squibb: Research Funding; Celgene: Research Funding; Merck: Research Funding; Abbvie: Research Funding; Amgen: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Regeneron: Research Funding; Sanofi: Research Funding; Takeda: Research Funding. Singh:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Tekle:Sanofi: Current Employment. Lin:Sanofi: Current Employment, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal