Introduction: Patients (pts) with advanced and heavily pretreated relapsed/refractory multiple myeloma (RRMM) have worse health-related quality of life (HRQoL) than those at earlier stages of the disease and have limited treatment options available to enable improvement in HRQoL, particularly those who are triple-class exposed. Talquetamab (tal), a novel GPRC5D×CD3 bispecific antibody, has shown deep and durable responses in the MonumenTAL-1 study (NCT03399799/NCT04634552) in pts with RRMM. Here, we report patient-reported outcomes (PROs) from the MonumenTAL-1 study, focusing on data through cycle 21 from the tal 0.4 mg/kg weekly (QW) dosing cohort (initial results presented at ASH 2022) and new data through cycle 15 from the tal 0.8 mg/kg every other week (Q2W) dosing cohort.
Methods: MonumenTAL-1 is a first-in-human, phase 1/2, single-arm study of tal monotherapy. Pts with triple-class exposed RRMM received the recommended phase 2 doses of tal (0.4 mg/kg QW or 0.8 mg/kg Q2W). PRO assessments included the following instruments: the European Organisation for Research and Treatment of Cancer quality of life questionnaire core 30 (EORTC QLQ-C30), a cancer-specific PRO instrument, with scores ranging from 0 to 100 (higher scores indicate better global health status [GHS] and functioning, whereas higher scores for symptoms represent worse severity, and a 10-point change from baseline indicates meaningful change); the EuroQol 5-Dimension 5-Level (EQ-5D-5L) visual analogue scale (VAS), which rates pt health from 0 (worst) to 100 (best); and the Patient Global Impression of Severity (PGIS) instrument, a single item that assesses severity of disease. PRO instruments were administered at screening, day 1 of cycle 1, then day 1 of every other cycle. Results for each instrument are presented for specific cycles that have a larger sample size (n≥30); later cycles presented for the QW cohort are due to longer follow-up duration (median,18.7 months) compared with the Q2W cohort (median, 12.3 months), for which earlier cycles are presented.
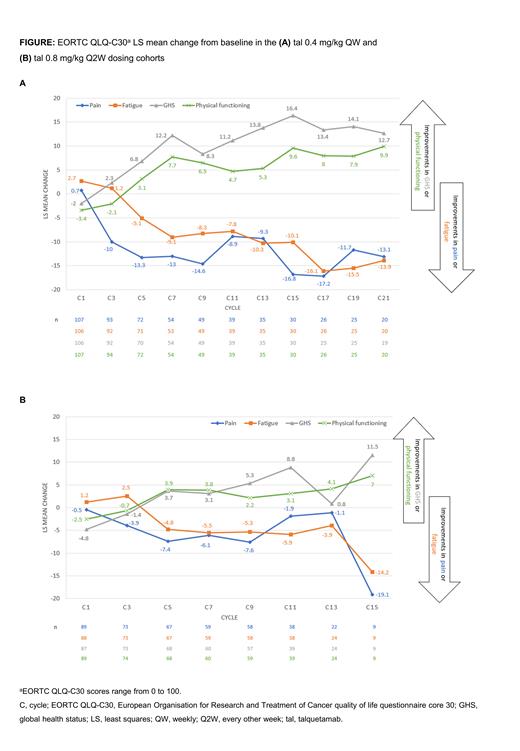
Results: As of January 17, 2023, phase 2 data were available for 122 and 109 pts who received ≥1 administration of tal QW or Q2W, respectively; compliance for completion of the EORTC QLQ-C30 was >97% at baseline and >80% at most post-treatment visits (in each cohort). Least squares (LS) mean change from baseline demonstrated clinically significant improvements in GHS (16.4 points QW, 8.8 points Q2W) and physical functioning (9.6 points QW, 3.1 points Q2W) at cycle 15 in QW and cycle 11 in Q2W cohorts, respectively, along with reductions in fatigue (-10.1 points QW, -5.9 points Q2W) and pain (-16.8 points QW, -1.9 points Q2W) symptoms at cycle 15 in QW and cycle 11 in Q2W cohorts, respectively (Figure). An increasing ability to engage in social roles and activities (role functioning) was also reported by pts in the QW cohort, with an 8.5-point improvement from baseline at cycle 15. Pts in the QW cohort reported meaningful improvement from baseline at later cycles; these improvements were particularly evident in GHS (57%) and social function (47%) at cycle 15. In the Q2W cohort, meaningful improvements from baseline in fatigue (45%) and emotional function (42%) were larger at cycle 7. Pts' global impression of their MM disease severity (PGIS) also improved from baseline, consistent with the meaningful improvement data: for example, in the QW cohort, ratings were 10.8% (very severe) and 40.8% (severe) at baseline compared with 0% and 6.7%, respectively, at cycle 15; in the Q2W cohort, ratings were 11.1% (very severe) and 34.3% (severe) at baseline compared with 2.8% and 19.4%, respectively, at cycle 11. Pts reported improvements from baseline in their health status (EQ-5D-5L VAS score), with LS mean change of 8.1 (95% CI, 2.6, 13.7) and 2.7 (95% CI
-2.3, 7.7) at cycle 9 in the QW and Q2W cohorts, respectively. Median time to improvement from baseline in most EORTC QLQ-C30 subscales and EQ-5D-5L VAS scores was ~2 months in both cohorts; estimates of median time to first worsening ranged from 2 to ~9 months.
Conclusions: Meaningful improvements from baseline in MM symptoms, physical function, and overall HRQoL were observed in tal QW and Q2W cohorts, with durable and short-term improvements, respectively. These favorable results in PROs add to the efficacy and safety profile of tal, highlighting tal as a compelling treatment option for pts with triple-class exposed RRMM.
Disclosures
Touzeau:Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Oriol:Menarini: Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Mateos:Amgen: Honoraria; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Honoraria; Stemline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS-Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; University of Salamanca/Gerencia Regional de Salud de Castilla y León: Current Employment. Rasche:Janssen: Consultancy, Honoraria; Amgen: Consultancy; GSK: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; Skyline Dx: Research Funding; Sanofi: Consultancy, Honoraria; Roche: Honoraria. Qin:Janssen: Current Employment. Kato:Janssen: Current Employment. Ming:Janssen-Cilag: Current Employment, Current equity holder in publicly-traded company. Katz:Janssen Pharmaceuticals, LLC: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Gries:Janssen Pharmaceuticals: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Campagna:Janssen: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Masterson:Janssen R&D: Current Employment, Current equity holder in private company. Hilder:Janssen: Current Employment, Current equity holder in private company. Tolbert:Janssen: Current Employment. Renaud:Johnson & Johnson: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Heuck:Janssen: Current Employment, Current equity holder in private company. Moreau:janssen, celgene BMS, abbvie, sanofi, amgen, takeda, pfizer: Honoraria, Other: advisory boards; GSK: Honoraria, Other: Advisory Board. San-Miguel:BMS: Other: Advisory Board; Abbvie: Consultancy, Other: Advisory Board; Amgen: Consultancy, Other: Advisory Board; Celgene: Other: Advisory Board; GSK: Other: Advisory Board; Haemalogix: Other: Advisory Board; Janssen-Cilag: Other: Advisory Board; Karyopharm: Other: Advisory Board; MSD: Other: Advisory Board; Novartis: Other; Takeda: Other: Advisory Board; Regeneron: Other: Advisory Board; Roche: Other: Advisory Board; Sanofi: Other: Advisory Board; SecuraBio: Other: Advisory Board. Rodríguez Otero:Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures; Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures; Amgen: Other: Honoraria for lectures; Regeneron: Other: Honoraria for lectures; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel grant; Roche: Consultancy. Chari:Sanofi: Other: Advisory Board; Karyopharm: Other: Advisory Board; Shattuck Labs: Other: Advisory Board; AbbVie: Other: Advisory Board; Millenium/Takeda: Consultancy, Research Funding; Seattle Genetics: Other: Advisory Board, Research Funding; BMS: Consultancy, Other: Advisory Board, Research Funding; Secura Bio: Consultancy, Other: Advisory Board; Amgen: Consultancy, Other: Advisory Board, Research Funding; Genentech: Other: Advisory Board; Janssen: Consultancy, Other: Advisory Board, Research Funding; Antengene: Consultancy; Glaxo Smith Kline: Other: Advisory Board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal