Overview
Given the rarity of children and adolescents with relapsed and refractory (r/r) B-cell Non-Hodgkin Lymphoma (B-NHL) and the large number of potential agents in development for adult mature B-cell malignancies, there is urgent need for prioritisation of agents as proposed by the ACCELERATE Paediatric Strategy Forum for medicinal development in paediatric B-NHL (Pearson 2019). The process developed for the global platform study Glo-BNHL (published separately) is described here.
Prioritisation process
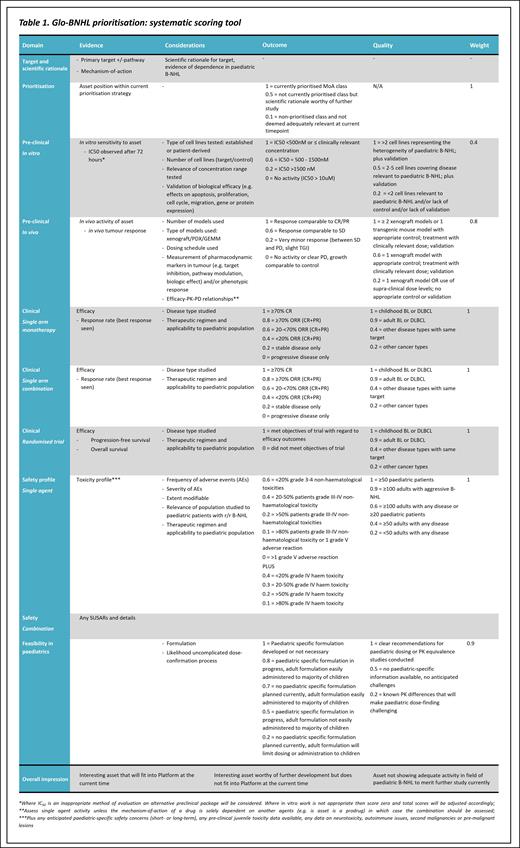
Assets submitted for review by industry collaborators are prioritised under Confidentiality Disclosure Agreements by the Glo-BNHL Trial Steering Committee (TSC) comprising expert clinicians, statisticians and patient representatives. The assessment includes a quantitative element addressing each of the following areas, with greater weighting given to more relevant criteria such as paediatric-specific and clinical data.
Target and scientific rationale
Asset position within current prioritisation strategy*
Pre-clinical data evaluating in vitro sensitivity, including the number, heterogeneity and relevance of cell lines tested and quality of the submitted elements of evidence
Pre-clinical data evaluating in vivo activity of the asset including the number, type and relevance of models and quality of the elements of evidence submitted
Clinical data regarding monotherapy efficacy in single arm and randomised trials, including the disease-type and therapeutic regimen studied
Clinical data regarding combination efficacy (if studied)
Clinical data regarding safety of the agent with regard to frequency, severity and modifiability of adverse events, including applicability to the paediatric population
Feasibility of delivery in paediatrics, including formulation and dose-confirmation processes
*Classes of novel agents prioritised for inclusion at the initiation of the trial were: (1) Bispecific antibodies (2) antibody-drug conjugates (3) chimeric antigen receptor T-cell products. The TSC has committed to updating this list as the field evolves.
The assessment also includes a qualitative element incorporating expert opinion regarding relevance of the target, mechanism of action, and overall impression of the asset. These elements are combined to inform the final discussion before a decision is made.
A formal written response from the Sponsor and TSC Chair outlining the TSC decision on whether the asset has been prioritised for evaluation within Glo-BNHL, and associated rationale, is issued to the relevant industry partner (now also copied to the European Medicines Agency (EMA)) within 28 days of receipt of the asset information pack. A follow-up clarification meeting is offered. For prioritised assets, following agreement from the relevant industry partner, the process of formal inclusion in Glo-BNHL then begins.
Scope of assets reviewed
Between September 2020 and June 2023, information packs for 7 assets were submitted for review from 6 pharmaceutical companies. The assets spanned 5 classes of drugs: 3 bispecific antibodies, 1 antibody-drug conjugate, 1 chimeric antigen receptor T-cell product, 1 small molecule inhibitor and 1 immunomodulator. Formal responses were issued within a median of 20 days (mean 21.3 days) followed by a clarification meeting on 5 occasions.
Prioritised assets
Four of the 7 reviewed assets were prioritised for inclusion within Glo-BNHL. At time of planned opening only 2 of these assets are included. The other 2 assets are being investigated in industry-led competitive trials. A further asset was deemed to be of potential interest but insufficient data were available due to the early stage of development to reach a final conclusion (further review pending).
Non-prioritised assets
The rationale for non-prioritisation of assets included irrelevant target in paediatric B-NHL, insufficiently convincing mechanism of action, and lack of superiority over other agents within a non-prioritised class. The EMA has subsequently waived the obligation to submit the results of paediatric B-NHL studies for both non-prioritised assets reasoning they do not represent potential for significant therapeutic benefit.
Summary
The Glo-BNHL international platform trial prioritisation process represents a model for handling the challenge of development of multiple potential agents in very rare populations in the global arena.
Disclosures
Gore:Amgen: Consultancy; Novartis: Consultancy; Roche: Consultancy. Minard-Colin:BMS: Consultancy; Adaptimmune Therapeutics plc: Consultancy; Roche: Consultancy; Aztra: Consultancy. Bollard:Cabaletta Bio, Catamaran Bio: Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: Patent applications in CAR-NKs; Roche: Consultancy. Allen:Sobi: Consultancy. Auperin:MSD France: Consultancy. Burke:Novartis: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal