Background: First-line FL treatments typically include an anti-CD20 monoclonal antibody with alkylator-based chemotherapy. Despite encouraging efficacy, most patients (pts) eventually relapse; novel therapies that improve outcomes and prolong remission in 1L FL are needed. Also, chemotherapy-containing regimens may have substantial toxicities, emphasizing the need for new therapies with improved safety. Mosunetuzumab (M), a CD20xCD3 bispecific antibody that redirects T cells to eliminate malignant B cells, has shown manageable safety with high complete remission rates in pts with relapsed/refractory (R/R) FL after ≥2 prior lines of therapy (Budde et al. Lancet Oncol 2022). Lenalidomide (Len), a potent immunomodulatory agent, may have synergistic effects with M. Initial results from the CO41942 Phase Ib/II trial (NCT04246086) showed that M (intravenous) combined with Len had a manageable safety profile and encouraging chemotherapy-free anti-lymphoma activity in pts with R/R FL who had ≥1 prior line of therapy (Morschhauser et al. ASH 2021). We present preliminary safety, efficacy, and biomarker data from this ongoing trial of M (subcutaneous [SC]) combined with oral Len in pts with 1L FL who require systemic therapy.
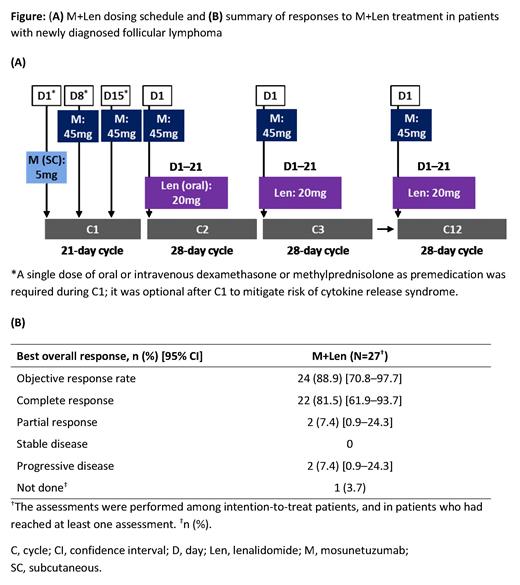
Methods: Pts with 1L FL requiring systemic therapy (investigator-assessed using GELF criteria) with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0-2 are included; target enrollment is 40 pts. Pts received 12 cycles of M+Len (cycle [C] 1, 21 days; C2-12, 28 days); in C1, 5mg of M was given on Day (D) 1, with the target dose (45mg) given on C1D8, C1D15, and on D1 of C2-12; Len (20mg) was given on D1-21 of C2-12 ( Figure A). Cytokine release syndrome (CRS) was reported using ASTCT criteria (Lee et al. Biol Blood Marrow Transplant 2019). Responses by PET-CT were investigator-assessed using Lugano 2014 criteria (Cheson et al. J Clin Oncol 2014). Flow cytometry (fluorescence-activated cell sorting) on whole blood was used to assess peripheral biomarkers.
Results: At clinical cutoff date (CCOD; May 2, 2023), 37 pts were enrolled: 21 (56.8%) had been on the study for 0-3 months, 12 (32.4%) for 3-6 months, and 4 (10.8%) for 6-9 months.
Median age was 62.0 (range 28-83) years and 20 (54.1%) pts were male. All pts had a baseline ECOG PS of ≤1; 32 (86.5%) had Ann Arbor stage III-IV; and 7 (18.9%), 13 (35.1%), and 17 (45.9%) had a FL International Prognostic Index score of 0-1 (low), 2 (intermediate), and 3-5 (high risk), respectively. All pts reported ≥1 treatment-emergent adverse event (TEAE); 12 (32.4%) had ≥1 serious TEAE. Two pts discontinued study treatment after the C1D15 dose, one pt due to uveitis and one pt due to tumor flare. TEAEs related to M or Len occurred in 36 (97.3%) and 29 (78.4%) pts, respectively. At least one Grade (Gr) 3-4 TEAE occurred in 16 (43.2%) pts, the most common being neutropenia (seven pts). No pts reported Gr 5 AEs. CRS occurred in 20 (54.1%) pts; all were Gr 1, except for one pt with Gr 2 CRS, and all events resolved (median CRS duration: 2 days [range 1-26]). CRS frequency was highest after C1D1 (32.4%), and steadily declined over subsequent days and cycles. No immune effector cell-associated neurotoxicity syndrome events were observed.
To date, 27 pts were efficacy evaluable ( Figure B). Of 24 (88.9%) responders, 22 (81.5%) had a complete metabolic response (21 achieved by the first response assessment at end of C3), and two (7.4%) had a partial metabolic response. One pt (3.7%) had no response assessment by CCOD, and two (7.4%) had progressive disease but were recognized to have biopsy-proven transformed FL during C1 and C2. All responses were maintained at CCOD.
Preliminary biomarker analyses of pt blood samples showed: increased CD69 and sustained HLA-DR expression in CD8 T cells, with modulation of CD8 subsets favoring central/effector memory phenotypes; sustained natural killer-cell activity; and minimal effects on CD4 T cells (except for lower PD-1 expression).
Conclusions: This fixed-duration, chemotherapy-free M+Len regimen offers a convenient means for outpatient SC administration, and has a manageable early safety profile with promising anti-lymphoma activity in pts with 1L FL requiring systemic therapy, based on the preliminary data. Advancing M+Len into the first-line setting offers potential benefits in chemotherapy-naïve pts. Safety, efficacy, biomarker, and pharmacokinetic data from the complete cohort (40 pts) will be presented.
OffLabel Disclosure:
Morschhauser:F. Hoffmann-La Roche Ltd, AbbVie, BMS, Genmab, Gilead, Novartis: Consultancy; F. Hoffmann-La Roche Ltd, Gilead, AbbVie: Membership on an entity's Board of Directors or advisory committees. Patel:AstraZeneca, Bristol Myers Squibb, Kite, TG Therapeutics: Speakers Bureau; Adaptive Biotechnelogies, AstraZeneca, Bristol Myers Squibb, CRISPR Therapeutics, Curis, Inc, Epizyme, Fate Therapeutics, Genentech, Inc. / F. Hoffmann-La Roche Ltd, Kite, Loxo Oncology, MEI Pharma, Merck, Nurix, Pharmacyclics/Janssen, Sunesis Pharmaceuti: Research Funding; Abbvie, ADC Therapeutics, AstraZeneca, BeiGene, Bristol Myers Squibb, Caribou Biosciences, Epizyme, Genentech, Inc. / F. Hoffmann-La Roche Ltd, Kite, Loxo Oncology, MEI Pharma, Merck, Morphosys, Nurix, Pharmacyclics/Janssen, Sana Biotechnology, TG Therape: Consultancy. Bobillo:AstraZeneca, Abbvie: Honoraria; Vall d'Hebron University Hospital: Current Employment. Cordoba:F. Hoffmann-La Roche Ltd, Takeda, Abbvie, Janssen, AstraZeneca, Lilly, BeiGene, BMS, Genmab, Incyte, Gilead: Speakers Bureau; European Hematology Association (EHA), Spanish Society Hematology (SEHH): Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd, Takeda, Abbvie, Janssen, AstraZeneca, Lilly, BeiGene, BMS, Genmab, Incyte, Gilead: Consultancy; Fundacion Jimenez Diaz University Hospital: Current Employment. Eyre:Loxo Lilly: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PeerView: Speakers Bureau; Medscape: Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Autolus: Consultancy; KITE: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eli Lilly and Company: Consultancy, Honoraria, Speakers Bureau; Loxo Oncology: Consultancy, Honoraria, Other, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Secura Bio: Membership on an entity's Board of Directors or advisory committees. Bishton:Abbvie: Consultancy; Celltrion, F. Hoffmann-La Roche Ltd, Takeda, Gilead: Honoraria; Lilly, F. Hoffmann-La Roche Ltd, Incyte: Membership on an entity's Board of Directors or advisory committees. Houot:Kite/Gilead, Novartis, Bristol-Myers Squibb/Celgene, ADC Therapeutics, Incyte, Miltenyi: Consultancy; Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda, F. Hoffmann-La Roche Ltd: Honoraria. Osborne:F. Hoffmann-La Roche Ltd, Takeda, Servier, Kite Gilead, MSD, Novartis, BeiGene, AstraZeneca, Syneos, Autolus, Kyowa Kirin, Abbvie, Incyte, BMS/Celgene, Janssen, Sobi: Consultancy; F. Hoffmann-La Roche Ltd, Takeda, Pfizer, Servier, Kite Gilead, MSD, Novartis, BeiGene, AstraZeneca, Syneos, Autolus, Kyowa Kirin, Abbvie, Incyte, BMS/Celgene, Janssen, Sobi: Honoraria; F. Hoffmann-La Roche Ltd, Takeda, Pfizer, Kite Gilead, MSD, Novartis, AstraZeneca, Kyowa Kirin, Abbvie, Incyte, BMS/Celgene, Janssen: Speakers Bureau. Gálvez-Carvajal:Medical Oncologist, Medical Oncology Intercenter Unit, Regional and Virgen de la Victoria University Hospitals: Current Employment. Thieblemont:Gilead Sciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Bayer: Honoraria; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Hospira: Research Funding; Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Cellectis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Paris University, Assistance Publique, hopitaux de Paris (APHP): Current Employment; Kyte, Gilead, Novartis, BMS, Abbvie, F. Hoffmann-La Roche Ltd, Amgen: Honoraria; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Janssen: Honoraria, Other: Travel Expenses. Yee:F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company; Genentech, Inc. / F. Hoffmann-La Roche Ltd: Current Employment. Knapp:F. Hoffmann-La Roche Ltd: Current Employment. Purev:F. Hoffmann-La Roche Ltd: Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment. Li:Hoffmann-La Roche Ltd (Canada): Current Employment. Chen:F. Hoffmann-La Roche Ltd / Genentech, Inc.: Current Employment; University of California, San Francisco (UCSF): Ended employment in the past 24 months. Banta:Genentech, Inc.: Current Employment. Sit:F. Hoffmann-La Roche Ltd: Current equity holder in private company; F. Hoffmann- La Roche Ltd: Current holder of stock options in a privately-held company. Bachy:Roche: Consultancy, Honoraria; Amgen: Research Funding; Bristol Myers Squibb: Honoraria, Other: Personal Fees, Research Funding; Novartis: Honoraria, Other: Personal Fees; Hospices Civils de Lyon Claude Bernard Lyon 1 University: Current Employment; Pfizer: Honoraria, Other: Personal Fees; Takeda: Honoraria; Incyte: Honoraria; Kite, a Gilead Company: Honoraria, Other: Personal Fees.
Mosunetuzumab (Lunsumio) is a bispecific CD20-directed CD3 T-cell engager indicated for the treatment of adult patients with relapsed or refractory FL after two or more lines of systemic therapy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal