Acute myeloid leukemia (AML) is a highly heterogeneous disease with a variable prognosis. The exact mediators of AML are not fully understood but there are several factors that may contribute to the development of the disease. These can be split into genetic and non-genetic causes. cytogenetic abnormalities have been identified such as FLT 3 gene which frequently mutated in AML and associated with a poor prognosis. As for non-genetic causes: prior exposure to chemotherapy or radiation therapy have been implicated. Lately cigarette smoking in AML has been shown to have poor survival outcome for AML patients relative to non-smokers.
Cigarette smoke contains many compounds, some of which have been associated with increased risk for AML. Benzene, radioactive components, and possibly other carcinogens are present in tobacco and tobacco smoke which lead to production of chromosomal defects. Cigarette smoke exposure globally alters DNA methylation in blood cells and these changes can persist for decades. All commercial tobacco products, including smokeless tobacco, cigarette smoke and E cigarettes contain tobacco-specific nitrosamines such asN′-nitrosonornicotine (NNN), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK). NNK and NNN promotes tumor growth by enhancing and deregulating cell proliferation, survival, migration, and invasion, thereby creating a microenvironment for tumor growth.
This calls for a need for to understand the molecular changes in AML especially when exposed to agents that can impact survival. Therefore, we hypothesize when FLT-3 AML model is exposed to smoking metabolites such as cigarettes smoking condensate (CSC), NNN, NNK and E cigarettes can lead to increased leukemia burden.
To test this in vitro CSC treatment of AML cells was used to evaluate how chemicals from cigarette smoke can impact AML cells without the high heat combustion from cigarettes. NNN and NNK treatment were separately used for treatment of AML cells. Two FLT-3 AML cell lines used (MOLM-13 and MV-4-11) and non-FLT-3 AML cell line (OCI-AML3) as control.Cells were treated in vitro with 10 ug/ml cigarette smoke condensate (CSC) or 1uM NNN or 1 uM NNK or vehicle (DMSO). Viability and cell proliferation were measured with each passage every 48-72 hours. There was no difference in cell proliferation and viability with CSC, NNN or NNK exposure. This suggests possibly that microenvironment is needed to evaluate the impact of smoking on AML progression.
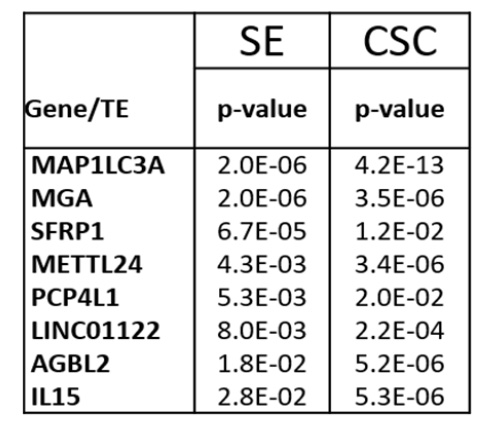
To test this in vivo, luciferase tagged MOLM13 cells were treated in vitro with 10ug/ml cigarette smoke condensate (CSC) or vehicle DMSO for two weeks then injected into mice. Mice injected with MOLM 13 cells treated with CSC had significantly more leukemia burden than control. Similar findings were replicated in MV-4-11 mouse model. Next, we wanted to evaluate if this increased leukemia burden leads to DNA methylation changes. We Found 8 genes to be significantly hypermethylated from the CSC two-week in vitro treatment. This was also found in the vivo experiment where mice were exposed to Cigarette smoking in vivo. (Table 1)
This study shows that CSC treated AML cell lines can lead to increase leukemia burden in vivo but not in vitro. There are difference in DNA methylation between AML cells lines exposed to CSC compared to control. Our future directions will include evaluate leukemia burden in FLT-3 AML mouse model exposed to NNN, NNK and E- cigarettes Study ROS changes, protein expression and DNA methylation impacted by smoking.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal