Introduction: Clonal hematopoiesis (CH) is a pre-malignant condition characterized by over-representation of blood cells derived from a single stem-cell clone. CH is strongly associated with aging with 10-20% of people over the age of 70 in various series reported to harbor a detectable clone. The most commonly mutated genes in aging-related CH are DNMT3A & TET2 followed by ASXL1 as a distant third and a smaller frequency of mutations in the splicing genes SF3B1, SRSF2, PRPF8 and U2AF1. CH portends a strong underlying risk for development of Myelodysplastic Syndrome (MDS) and other hematologic neoplasms as well as future cardiovascular disease and all-cause mortality. While much is known about these pre-leukemic states that confer risk for developing AML, most of the published studies are based on predominantly white Europeans. There has also recently been an effort to provide genomic and clinical services to high-risk individuals (CH clinics) to predict their pre-leukemic risk, however almost all these services are unavailable to underserved, ethnic minority populations. We decided to query the rate and associations of CH in diverse populations of New York City (NYC). The few published studies investigating CH in diverse populations have used self-identified categorization of race and ethnicity. However, these groupings can be too general and inaccurate when investigating genetic variation, particularly in diverse and multi-ethnic populations. Here we present our CH analysis on the multi-ethnic All of Us (AoU) cohort (version 6) for NYC individuals. A population genetics approach was used to identify groups of individuals who share genetic segments identical by descent, which corresponded to several known communities. We estimated the frequency of CH among the populations identified through this approach.
Methods & Results:
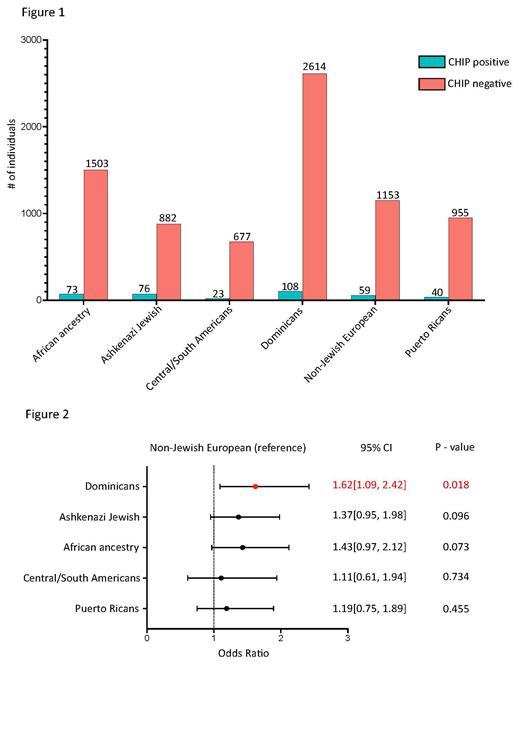
Of a total of 8538 NYC individuals in the AoU cohort that had information on lifestyle (smoking), 393 (4.64%) had CH. We assigned these individuals to 11 genetic ancestry groups based on sharing of genomic segments identical by descent. Out of these 11 groups, 6 had >20 individuals with CH, so we focused on those groups for further analysis (Figure 1). We constructed a logistic regression model between CH status and ancestry taking into account age, age*age (to account for a non-linear effect), sex, smoking and mean BMI with individuals belonging to the ‘Non-Jewish European ancestry group’ as a reference. We found that Dominicans have a higher rate of CH in comparison to other ancestry groups (OR: 1.62; p-value: 0.018; Figure 2). Dominicans as an ancestry group independently showed statistically significant associations with cardiovascular diseases such as heart failure, cardiac arrythmia and cardiomegaly. Whether this association is related to elevated CH remains to be investigated. We also measured the enrichment of variants in individual CH genes in each ancestry group, keeping all other individuals as a reference. Our results showed that CH in individuals belonging to the African ancestry group, as well as the Ashkenazi Jewish group have significantly higher rates of somatic mutations in DNMT3A (OR: 1.82; p-value: 0.027) and TET2 (OR: 2.36; p-value 0.004), respectively, compared to the other populations.
Conclusions:
Leveraging population genetic-based inference of ancestry groups, we have demonstrated for the first time that different ancestries residing in NYC have a different risk and pattern of CH somatic mutations. In particular, we demonstrate that non-Jewish Europeans have lower risk of CH compared to all other groups, and Dominicans in particular have a significantly higher risk. Understanding the relative rates of CH across ancestries and their association with chronic diseases such as CVD and cancer will further refine recommendations directed towards lifestyle modifications and rational therapeutics that prevent the progression of CH & associated comorbidities.
Disclosures
Verma:Novartis: Consultancy; Acceleron: Consultancy; Bakx: Consultancy, Current equity holder in private company; Janssen: Honoraria; Curis: Research Funding; GSK: Research Funding; Incyte: Research Funding; Medpacto: Research Funding; Eli Lilly: Research Funding; Stelexis: Consultancy, Current equity holder in private company, Honoraria; Throws Exception: Current equity holder in private company; Bristol Myers Squibb: Research Funding; Celgene: Consultancy; Prelude: Research Funding. Konopleva:Reata Pharmaceuticals.: Current holder of stock options in a privately-held company, Patents & Royalties; AbbVie, Forty Seven, Precision Biosciences, Gilead Sciences, Genentech, Janssen, Sanofi, MEI Pharma, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini.: Consultancy; Abbvie, Allogene Therapeutics, Cellectis, Forty Seven, Gilead Sciences, Genentech, Sanofi, MEI Pharma, Rafael Pharmaceuticals, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini, Precision BioSciences.: Research Funding. Shastri:Gilead Sciences: Membership on an entity's Board of Directors or advisory committees; Kymera Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal