BACKGROUND:
β-Thalassemia is an inherited hemolytic disease that is prevalent worldwide. Over 200 mutations in the HBB gene, which encodes adult hemoglobin (HbA), result in β-thalassemia. Hereditary persistence of fetal hemoglobin (HbF) can alleviate the symptoms of anemia. CRISPR-Cas9-mediated disruption of the BCL11A erythroid enhancer results in the reduction of BCL11A expression and the induction of fetal γ-globin, which is a practicable therapeutic strategy for treating transfusion-dependent β-thalassemia (TDT).
METHODS:
We obtained mobilized autologous CD34+ cells from 4 TDT patients sponsor initiated in phase I/II clinical trial (NCT05577312) and 6 TDT patients in investigator initiated clinical study(NCT04211480, NCT04205435)respectively. These cells were edited with CRISPR-Cas9 RNP at the +58 erythroid specific enhancer region of the BCL11A gene and then reinfused after the patients had undergone myeloablative busulfan conditioning. We subsequently monitored adverse events, neutrophil and platelet engraftment. Efficacy assessments included proportion of transfusion-independent (TI) subjects, levels of total hemoglobin and HbF, and proportion of red blood cells expressing HbF in peripheral blood. TI was evaluated for elimination of transfusions starting 42 days after the last transfusion and Hb above 90 g/L.
RESULTS:
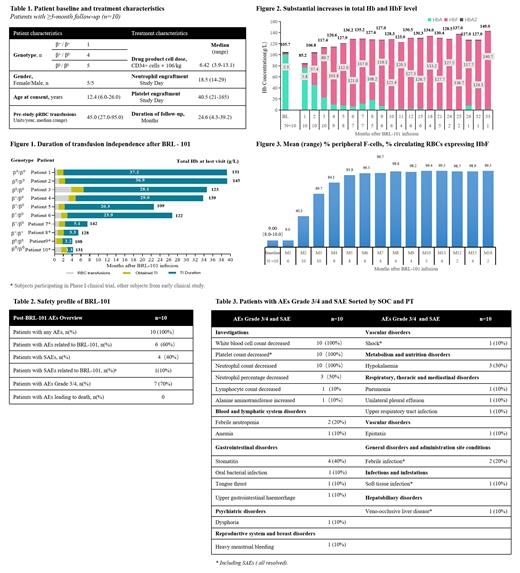
Between October 15, 2019 and November 20, 2022, 10 patients with TDT were enrolled and received BRL-101 with a median age of 12.4 years (6-26). Among all the treated patients, 5 patients were β 0/β 0 phenotype, 4 patients were β0/β+ phenotype and 1 patient were β +/β + phenotype. As of July 20, 2023, the median follow- up was 24.6 months (4.3-39.2 m) (Tab.1). All of 10 patients achieved TI. The median duration of TI was 22.1 months (1.3 - 37.2 m). The longest duration of TI was 37.2 months (Fig.1). The levels of HbF ranged from 2.3 to 140.7g/L and the levels of total hemoglobin ranged from 105.7 to 149 g/L (Fig.2). At 6 months after BRL-101 infusion, the proportion of HbF-expressing red blood cells in peripheral blood had reached 96.5%, and then continued to rise and remained around 98-99% (Fig.3). Treatment-related adverse events were typical of those associated with myeloablation and autologous stem-cell transplantation, mainly manifested as hematological toxicities (Tab.2, Tab.3). 3 patients experienced adverse events (AEs) grade ≥ 3 which related to BRL-101. 4 patients experienced serious AEs (SAEs), including decreased platelet count, shock, febrile infection, soft tissue infection, and veno-occlusive liver disease. Only decreased platelet count may related to BRL-101, the others were due to busulfan treatment. All the SAEs were resolved. No study drug-related withdrawals or deaths occurred during treatment.
CONCLUSIONS:
Whether genotype was β 0/β 0 or non-β 0/β 0, BRL-101 demonstrated clinically meaningful increases in total Hb and HbF which occurred early and have been maintained over time, the safety profile of BRL-101 is generally consistent with that of myeloablative conditioning and autologous hematopoietic stem cell transplant. The updated data with 10 patients reported here are consistent with previous reports and support continued investigation of BRL-101 as a potential functional cure for patients with TDT.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal