Introduction: Monitoring of chimeric antigen receptor (CAR) transgene levels in peripheral blood post tisagenlecleucel (tisa-cel) infusion provides information on expansion and persistence of CAR T-cells. In adult diffuse large B cell lymphoma (DLBCL) and follicular lymphoma (FL) patients (pts), the expansion of CAR T-cells was similar between responding and non-responding pts, while in pediatric and young adult acute lymphoblastic leukemia (ALL) pts, higher expansion in responding pts was observed. Based on long term follow up data from tisa-cel treated pts, analyses were performed to delineate the impact of CAR persistence and B cell aplasia on duration of remission (DOR).
Methods: Transgene levels in blood measured by quantitative polymerase chain reaction were available from pivotal phase II studies in pts with r/r ALL (ELIANA [N=79], ENSIGN [N=64], NCT03123939 [N=69], NCT01626495 [N=60]), r/r DLBCL (JULIET [N=115]), and r/r FL (ELARA [N=97]), along with the long term follow up study (NCT02445222). The time related to loss of persistence of CAR T-cells (T loss) was defined as the time at which transgene levels first dropped below 50 transgene copies/µg DNA (approximates to lower limit of quantification (LLOQ) of the assay) after maximal expansion. Kaplan Meier analysis was performed to investigate the impact of duration of T loss and time corresponding to last quantifiable level (T last) on DOR/relapse. The impact of time to B cell recovery (>1% CD19+ B cells/WBCs or >3% CD19+ B cells/lymphocytes for ALL, and 80-616 cells/µL for DLBCL and FL), on DOR/relapse was also investigated.
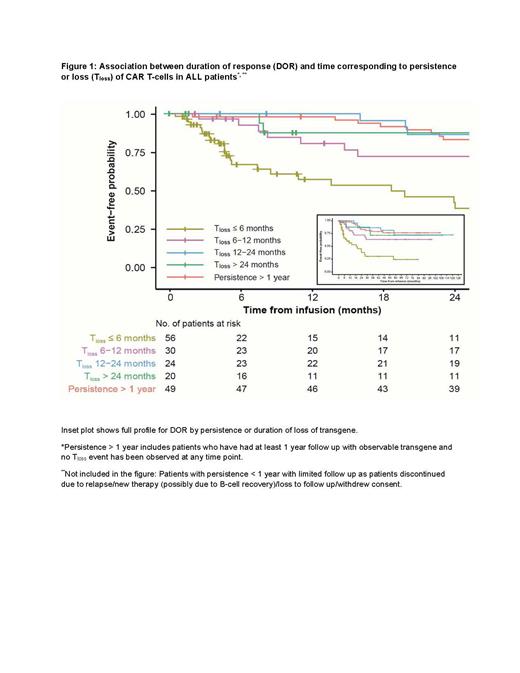
Results: Long term CAR persistence in tisa-cel treated pts has been observed for up to 9, 6, and 2.5 years for ALL, DLBCL, and FL pts, respectively, reflecting differing length of follow up based on initiation of trials in respective indications. Across indications, pts who lost transgene within 6 months or between 6-12 months had shorter DOR relative to pts with persistent transgene (Fig 1, ALL pts). In ALL, the median T loss was 27.4, 18.2, 9.7, and 18.0 months for ongoing complete response (CR) > 12 months, CR pts between 6-12 months, relapsed pts < 12 months, and relapsed pts > 12 months, respectively. However, of the pts who lost transgene within first 6 months, some pts maintained durable responses for ≥12 months (29%, 15%, and 50% in ALL, DLBCL, and FL, respectively). Among ALL relapsed pts, majority (17/26, 65%) of the pts with T loss < 6 months showed B cell recovery, however 6 pts had T loss at < 6 months as well as B-cell recovery but maintained response for ≥ 12 months. In ALL, Pulsipher et al.,(Blood Cancer Discov. 2022)have shown that NGS MRD within the first 6 months to 1 year post infusion may be a more reliable predictor of potential relapse than B cell recovery. The median time to B-cell recovery was 266 days in pts who relapsed/censored within 12 months but was not reached for pts with ongoing response at 12 months. On the contrary, B cell recovery seems to have no association with relapse in DLBCL or FL pts.
Of the ALL pts with CAR persistence, pts with <50% morphologic blasts at any time prior to infusion of tisa-cel had longer DOR compared to pts with ≥50% blasts. This may also reflect a more resistant high risk ALL at the time of study entry or greater potential for stochastic loss of CD19 in pts with the higher disease burden.
Conclusions: Long term sustained remission/response was observed in tisa-cel treated pts in the pivotal trials. The analyses demonstrate a positive association between CAR persistence and durable clinical responses across indications, however, some pts maintained durable responses despite early loss of transgene and/or early B cell recovery (< 6 months post infusion; Myers et al., J Clin Oncol. 2021). To note, in DLBCL and FL, the transgene levels in blood may not represent the levels at target sites including lymph nodes, moreover, T last and T loss are dependent on the duration of follow up, LLOQ etc.
Of note, these analyses represent data from pivotal tisa-cel clinical studies with a heavily treated pt population post multiple lines of therapy including prior HSCT, and may not represent pts treated with commercial product, at earlier times post diagnosis in current studies with fewer lines of prior treatment, lower pre-infusion disease burden, and/or other CAR product. In pts with early loss of transgene, further research is needed to identify potential factors or patient characteristics resulting in durable responses.
Disclosures
Awasthi:Novartis Institutes for BioMedical Research: Current Employment. Waldron:Novartis Pharmaceuticals Corporation: Current Employment. Grupp:Novartis: Consultancy, Research Funding; Vertex: Consultancy, Research Funding; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Juno: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cabaletta: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite: Research Funding; Adaptimmune: Consultancy, Membership on an entity's Board of Directors or advisory committees; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees; CBMG: Consultancy, Membership on an entity's Board of Directors or advisory committees; Servier: Research Funding. Pulsipher:Cargo Therapeutics: Membership on an entity's Board of Directors or advisory committees; Equillium: Membership on an entity's Board of Directors or advisory committees; Medexus: Membership on an entity's Board of Directors or advisory committees; Vertex: Membership on an entity's Board of Directors or advisory committees; Bluebird: Membership on an entity's Board of Directors or advisory committees; Gentibio: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Bader:Novartis: Consultancy, Research Funding; Neovii: Research Funding; BMS: Research Funding; Medac: Consultancy, Patents & Royalties: medac, Research Funding. Schuster:Novartis: Consultancy, Honoraria, Patents & Royalties; Mustang Bio: Consultancy; Incyte: Consultancy; Caribou Biosciences: Consultancy; Merck: Research Funding; Genentech/Roche: Consultancy, Research Funding; Takeda: Honoraria; Genmab: Consultancy; Kite Pharma: Consultancy; Nordic Nanovector: Consultancy; MorphoSys: Consultancy; Legend Biotech: Consultancy. Maziarz:Orca Therapeutics: Research Funding; Gamida: Research Funding; Kite: Consultancy; AlloVir: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Athersys: Other: Patent holder. Waller:Secura: Research Funding; CSL Behring: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; CRISPR: Consultancy; Allovir: Consultancy; Verastem: Consultancy, Research Funding; NCI R01: Research Funding; Sanofi: Research Funding; BMS: Research Funding; ORCA: Research Funding; PartnersTherapeutics: Research Funding; Cambium Medical Technologies: Current equity holder in private company, Other: Founder; Cambium Oncology: Current equity holder in private company, Other: Founder. Jaeger:Innovative Medicines Initiative 2 Joint Undertaking: Research Funding; BMS, Novartis, Gilead, Miltenyi, Janssen and Roche: Honoraria. Thieblemont:Bayer: Honoraria; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Janssen: Honoraria, Other: Travel Expenses; BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Paris University, Assistance Publique, hopitaux de Paris (APHP): Current Employment; Gilead Sciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Kite: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Hospira: Research Funding; Cellectis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kyte, Gilead, Novartis, BMS, Abbvie, F. Hoffmann-La Roche Ltd, Amgen: Honoraria; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding. Dreyling:Abbvie, Astra Zeneca, Beigene, BMS/Celgene, Gilead/Kite, Janssen, Lilly/Loxo, Novartis, Roche: Other: Scientific advisory boards; Abbvie, Bayer, BMS/Celgene, Gilead/Kite, Janssen, Roche: Research Funding; Astra Zeneca, Beigene, Gilead/Kite, Janssen, Lilly, Novartis, Roche: Honoraria. Fowler:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Research Funding; BostonGene, Corp.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Maier:Novartis Pharma AG: Current Employment. Willert:Novartis Pharmaceuticals Corporation: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal