Background: Hypomethylating agents (HMAs) (5-azacytidine or decitabine) combined with venetoclax (Ven) have become the new standard of care for patients with acute myeloid leukemia (AML) who are unfit for intensive chemotherapy. However, myelosuppression and infectious complications are common adverse events of currently approved HMA/Ven regimens, requiring dose interruptions/delays that often undermine treatment goals. Previous studies have identified the minimum dose of decitabine needed to deplete DNA methyltransferase-1 (DNMT1) without causing cytotoxicity. Administration of a single venetoclax dose synergizes with low dose weekly decitabine by inhibiting de-novo pyrimidine synthesis - a major mechanism of resistance to HMAs. A recently reported retrospective experience has shown proof of concept for a regimen of once weekly low dose decitabine combined with a single weekly dose of venetoclax in MDS and AML. Here, we report the feasibility portion of an ongoing Phase II clinical trial evaluating tolerability and efficacy of a metronomic dosing of decitabine and venetoclax for treatment of older patients with AML.
Aims: The primary endpoint of this study is percentage of patients who are able to continue on treatment during the 12-week induction period without dose interruptions or delays.
The key secondary endpoints include best overall response, rate of Grade >3 adverse events, infections, and hospitalizations. Correlative studies evaluate DNMT1 depletion in bone marrow at the time of diagnosis and at week 12.
Methods: This is a single arm open-label Phase II clinical trial (NCT05184842). Patients >18 years old with a diagnosis of AML, MDS, CMML, MDS/MPN, and MDS/AML not fit for intensive chemotherapy are eligible. Subcutaneous decitabine and oral venetoclax are administered on the same day, once a week at 0.2mg/kg and 400mg, respectively. A bone marrow exam and measurable residual disease (MRD) assessment by multiparametric flow cytometry (MFC) are performed following 12-week induction period.
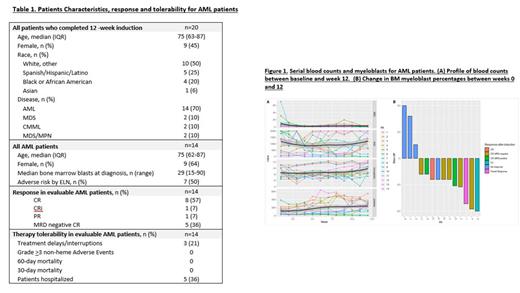
Results: Between June 2022 and April 2023, twenty patients on this study have completed the 12-week induction period, these included AML (n=14), MDS (n=2), MF (n=1), MDS/MPN (n=1), CMML (n=2). Median age was 75 and 50% of patients were minorities (Table 1). Seventeen patients (85%) were able to complete all 12 weeks of induction treatment without dosing interruptions or delays and only one patient (5%) had a dose interruption post-induction. There were no Grade > 3 non-heme therapy-related adverse events. Eight patients (40%) required hospitalization during the induction period and no patient required hospitalization post-induction. Of the 14 AML patients who have completed the 12-week induction period, the median age was 75 years old (63-87). At the end of induction, the ORR was 71% with 8 patients (57%) achieving a CR, 1 CRI and 1 PR; 5/8 CRs were MRD negative by MFC. At a median followup of 10.9 months, 7/14 (50%) responses are ongoing and 11/14 patients (79%) remain alive. Of the six patients with TP53 mutations, four achieved MRD-negative CR with transfusion independence and two with no response (one post-MPN AML and other with erythroid leukemia). During the induction period, four patients (29%) had progression of disease (POD). The primary refractory cases included one patient with post-MPN AML, one patient with FLT3-ITD and two patients with monocytic-M5 AML with RAS mutations. One of two patients with MDS, had an MRD negative CR and achieved transfusion independence while the other transformed to erythroid leukemia. Of the two patients with CMML, one had a CR with transfusion independence, and one with stable disease. The 30 and 60-day mortality was zero. Studies evaluating DNMT1 staining on bone marrow samples from patients treated on this study are ongoing.
Summary/Conclusion: In this preliminary analysis we demonstrate safety and efficacy of weekly, low-dose, metronomic dosing of decitabine and venetoclax, by allowing frequent sustained drug exposure often not possible with standard HMA/Ven regimens. The minimal cytotoxic nature of this regimen allowed for blood count recovery without drug interruptions with a high rate of clinical responses. (Figure 1). Additional cohorts are being enrolled in an expansion phase of the study in order to more fully assess the efficacy of this regimen in subsets of AML and MDS patients, including patients with p53 mutations.
Disclosures
Mantzaris:Kite, a Gilead company: Honoraria. Shastri:Gilead Sciences: Honoraria; Rigel Pharmaceuticals: Honoraria; Kymera Therapeutics: Honoraria, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria. Gritsman:iOnctura: Research Funding; ADC Therapeutics: Research Funding. Verma:Bakx: Consultancy, Current equity holder in private company; Janssen: Honoraria; Incyte: Research Funding; GSK: Research Funding; Curis: Research Funding; Acceleron: Consultancy; Novartis: Consultancy; Eli Lilly: Research Funding; Medpacto: Research Funding; Bristol Myers Squibb: Research Funding; Stelexis: Consultancy, Current equity holder in private company, Honoraria; Throws Exception: Current equity holder in private company; Celgene: Consultancy; Prelude: Research Funding. Konopleva:Reata Pharmaceuticals.: Current holder of stock options in a privately-held company, Patents & Royalties; Abbvie, Allogene Therapeutics, Cellectis, Forty Seven, Gilead Sciences, Genentech, Sanofi, MEI Pharma, Rafael Pharmaceuticals, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini, Precision BioSciences.: Research Funding; AbbVie, Forty Seven, Precision Biosciences, Gilead Sciences, Genentech, Janssen, Sanofi, MEI Pharma, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini.: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal