Introduction: Little is known about the target-cell intrinsic features that affect tissue susceptibility to T-cell mediated cell death. The major histocompatibility complex (MHC) Class I presents antigens to CD8+ T cells, while MHC class II presents antigens to CD4+ T cells, and this process is critical for infectious, tumor, auto- and allo -immunity. However, MHC Class I has been suggested to have a non-canonical role iron metabolism, but whether it plays a role in tissue tolerance to CD4+ T cell mediated damage is not known. Herein, we investigated the role of MHC Class I on target cells in CD4+ T-cell mediated damage to intestinal epithelial cells (IECs) utilizing clinically relevant models of T-cell mediated gastrointestinal damage from Graft-versus-Host Disease (GVHD), a major complication of allogeneic stem cell transplantation.
Methods: Because MHC class I is expressed on all nucleated cells, to specifically assess the role of MHC Class I on IECs, we generated and utilized intestine-specific MHC Class I knockout mice (B2m∆IEC) as recipients for MHC Class II-disparate bm12–>B2m∆IEC allogeneic stem cell transplant (allo-SCT). We utilized flow cytometry, immunological, molecular and biochemical assays to assess cell death pathways and tissue damage in IECs. We performed mechanistic studies to assess the role of iron and utilized clinically relevant iron chelation treatment to assess survival after treatment.
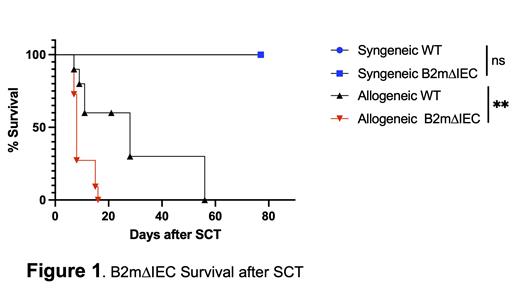
Results: Here, we demonstrate that absence of MHC Class I on target cells exacerbates CD4+ T cell mediated killing of IECs leading to greater mortality in the KO animals than WT recipients (P<0.01) (Fig.1). Compared with wild-type (WT) mice, B2m∆IEC mice, despite an increase in mortality following MHC II disparate allo-SCT, did not demonstrate significant changes in T cell activation, or antigen presentation. In vitro co-culture cell killing assays demonstrate that B2m knockout cells have increased cell death compared with WT cells when co-cultured with activated bm12 CD4+ T cells. Because B2m knockout mice have increased liver iron deposition, we hypothesized that the increase in GI damage might be a consequence of iron related cell death in the IECs in B2m∆IEC mice. We demonstrate that absence of B2m on the intestine is sufficient to drive an increase in tissue iron levels in the liver and intestine. Furthermore, B2m∆IEC allo-recipient mice demonstrated increased intracellular labile iron in IECs. Given the increased mortality in B2m∆IEC allo-recipient mice and the increase in intracellular iron in IECs, we investigated the role of iron-dependent cell death, ferroptosis. We show that IECs from B2m∆IEC allo-recipient mice have increased ferroptosis compared with WT animals (P<0.05). Finally, chelation of iron with administration of clinically utilized iron chelator (Deferasirox) ameliorated excess mortality from GVHD in the B2m∆IEC mice.
Conclusion: Absence of MHC Class I expression on target cells exacerbates CD4+ T cell mediated killing by enhancing iron dependent ferroptosis. These data demonstrate that in contrast to immunological paradigm, class I regulates CD4+ T cell mediated cytotoxicity even as it is essential for CD8+ T cell immunity.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal