Background:
Up to 30% of newly diagnosed myeloma (NDMM) patients have high-risk disease characterized by increased proliferation, resistance to apoptosis, focal and bone marrow-independent growth, and intraclonal heterogeneity. High-risk can be defined by fluorescence in situ hybridization (FISH) features including 17p deletion, translocation (t)(4;14), t(14;16), or t(14;20), and gain of 1q. However, some standard risk patients also exhibit high-risk characteristics, indicating that identifying novel targetable mediators of this phenotype is of interest.
Methods:
We conducted single cell RNA-Sequencing (scRNA-Seq) of CD138 + monoclonal/neoplastic and polyclonal/normal plasma cells (PCs) from 72 individuals across the plasma cell dyscrasia spectrum and 3 healthy controls to identify differentially expressed genes associated with myeloma pathobiology. Also, we leveraged publicly available gene expression profiling (GEP) databases, and performed pre-clinical studies both in vitro and in vivo.
Results:
CLPP was expressed with increased frequency in NDMM and relapsed/refractory myeloma patients compared to the precursors monoclonal gammopathy of undetermined significance and smoldering myeloma (GSE6477; p=4.03x10 -14). Notably, CLPP is the proteolytic component, along with CLPX, of the CLP complex which cleaves peptides and proteins in an ATP-dependent manner and is associated with the inner mitochondrial membrane. The GEP data were confirmed in our scRNA-Seq database, which showed CLPP over-expression occurred preferentially in each patient's monoclonal versus their own polyclonal PCs. Moreover, analysis of the Broad Institute Cancer Cell Line Encyclopedia revealed MM cells had the highest mean CLPP expression of any tumor cell type. In the NDMM CoMMpass database, high CLPP levels were associated with markers of increased disease burden (beta-2-microglobulin (p<0.001), calcium (p=0.0027), circulating plasma cells (p<0.001), marrow plasmacytosis (p<0.001), and lactate dehydrogenase (p=0.0025)). Moreover, these patients showed a significantly shorter progression-free and overall survival (both p<0.0001), and did not cluster in any of the Arkansas GEP70-defined myeloma subtypes.
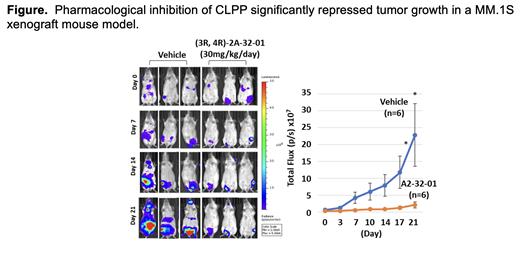
In MM.1S and H929 myeloma cells, specific CLPP knockdown with an inducible shRNA decreased cell proliferation and activated apoptosis as shown by increased Annexin V positivity. Similarly, pharmacologic CLPP inhibition produced a time- and concentration-dependent induction of apoptosis in a panel of cell lines representing myeloma molecular subtypes and drug resistant phenotypes. Ectopic CLPP overexpression promoted myeloma organoid growth, while its suppression had the opposite effect. Functional analysis revealed that CLPP knockdown or inhibition impaired cell proteolytic activity, mitochondrial transmembrane potential, and reduced adhesion to stromal cells while inducing reactive oxygen species and autophagy. To further pursue the underlying molecular mechanisms, we first examined the net downstream effects of CLPP knockdown and inhibition by mass spectrometry followed by mapping to the KEGG database. Recurrently up-regulated pathways included the mitochondrial unfolded protein response (UPR mt), lysosome transport, and autophagy utilizing and metabolic processes. In contrast, down-regulated pathways included oxidative phosphorylation, c-Myc signaling, RNA polymerase I-directed transcription, DNA replication and elongation, and the G2M checkpoint. Next, our metabolomics analysis revealed a significant attenuation of the Warburg effect in myeloma cells upon CLPP inhibition consistent with reduced glycolysis and pentose phosphate pathway activity. Notably, mitochondrial ATP production was significantly reduced, as confirmed by a seahorse XF Real-time ATP Rate assay. Finally, in vivo experiments demonstrated that pharmacologic CLPP inhibition impaired myeloma growth in an MM.1S-based xenograft model ( Figure).
Conclusions:
These pre-clinical data support the hypothesis that CLPP represents a novel mediator of high-risk behavior in multiple myeloma, demonstrate that its targeting results in mitochondrial dysfunction that attenuates myeloma growth and survival, and support development of pharmacologic CLPP inhibitors to target myeloma.
Disclosures
Lee:Regeneron: Consultancy, Research Funding; Pfizer: Consultancy; Amgen: Research Funding; Sanofi: Consultancy; Allogene Therapeutics: Consultancy; Takeda: Consultancy, Research Funding; GlaxoSmithKline: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Genentech: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding. Patel:Takeda: Consultancy; AbbVie; Allogene Therapeutics, Inc.; Arcellx; Bristol Myers Squibb/Celgene Corporation; Cellectis; Janssen Pharmaceuticals, Inc.; Nektar Therapeutic; Poseida Therapeutics; Precision BioSciences, Inc.; and Takeda Pharmaceuticals U.S.A., Inc.: Research Funding; AbbVie; Arcellx, AstraZeneca; Bristol Myers Squibb/Celgene Corporation; Caribou Science; Cellectis; Curio Bioscience; Genentech; Janssen Pharmaceuticals, Inc.; Karyopharm; Legend Biotech; Merck & Co., Inc.; Oncopeptides; Pfizer; Precision BioSciences: Consultancy. Symer:10x Genomics, Inc.: Current Employment, Current equity holder in publicly-traded company, Ended employment in the past 24 months. Manasanch:A: Consultancy; G: Consultancy. Orlowski:AbbVie, Adaptive Biotech, Asylia Therapeutics, Inc., BioTheryX, Bristol-Myers Squibb Pharmaceuticals, Karyopharm Therapeutics, Meridian Therapeutics, Monte Rosa Therapeutics, Nanjing IASO Biotherapeutics, Neoleukin Corporation, Oncopeptides AB, Pfizer, In: Consultancy, Honoraria; Asylia Therapeutics, BioTheryX Inc., Heidelberg Pharma: Other: Laboratory Research Funding, Research Funding; Asylia Therapeutics: Current equity holder in private company, Patents & Royalties; BMS/Celgene Corporation, CARsgen Therapeutics, Exelixis Inc., Heidelberg Pharma, Janssen Biotech Inc., Sanofi/Genzyme, Takeda Pharmaceuticals USA Inc.: Other: Clinical Research Funding, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal