Background: The majority of patients (pts) discontinue covalent (c) Bruton tyrosine kinase (BTK) inhibitors (BTKi) for either progression or intolerance. BTK Cysteine 481 substitution is known to contribute to cBTKi acquired resistance to ibrutinib, acalabrutinib, and zanubrutinib.Pirtobrutinib, a highly selective, non-covalent (reversible) BTKi has favorable oral pharmacology that enables continuous BTK inhibition throughout the daily dosing interval regardless of intrinsic rate of BTK turnover. Pirtobrutinib has demonstrated broad efficacy in pts with chronic lymphocytic leukemia (CLL) following prior therapy, including those treated with a prior cBTKi, independent of BTK C481 mutational status (Mato et al, NEJM, 2023). Here we report the largest systematic evaluation of genomic evolution in pts with CLL treated with pirtobrutinib conducted to date, using a larger cohort of pts and longer follow-up than previously reported.
Methods: Relapsed cBTKi pre-treated CLL pts in the phase 1/2 BRUIN trial (NCT03740529) who subsequently developed disease progression (PD) on pirtobrutinib monotherapy were included. Targeted next-generation sequencing (NGS) was centrally performed on peripheral blood mononuclear cells collected at baseline and within four months of PD .
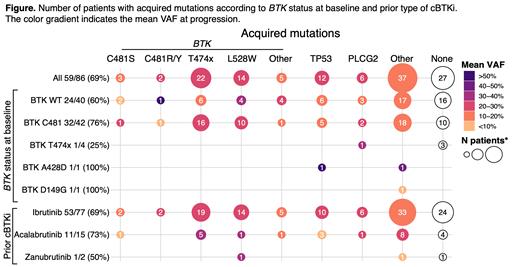
Results: As of May 5, 2023, 86cBTKi pre-treated CLL pts had paired NGS data available at baseline and pirtobrutinib progression. In this group, the median age was 69 years (range, 36-86), median number of prior lines of therapy was 4 (range, 1-10), 74 pts (86%) had discontinued prior cBTKi due to PD and 12 pts (14%) discontinued due to toxicity/other. Pts received one or more of the following prior cBTKi: ibrutinib (n=77, 90%), acalabrutinib (n=15, 17%), or zanubrutinib (n=2, 2%). Median time on pirtobrutinib treatment was 16 months (range, 1.2-39 months). Among these 86 pts who ultimately progressed on pirtobrutinib, the ORR (including partial response with lymphocytosis) was 83% (95%CI, 73-90). The most common baseline alterations were mutations in BTK (53%), TP53 (48%), SF3B1 (35%), ATM (23%), NOTCH1 (20%), XPO1 (16%), PLCG2 (14%), and BCL2 (9%). In 46 pts, 64 BTK mutations were detected at baseline and included C481S (n=45), C481F/R/Y (n=11), T474I/F/S (n=6), A428D (n=1), D149G (n=1). Among 42 pts with C481, BTK C481 VAF decrease or complete clearance was observed at PD in the majority of pts (86%, 36/42, complete clearance = 55%, 23/42). NGS of samples at PD showed 69% (59/86) of pts acquired ≥1 mutation. In 38 (44%) pts, 52 acquired BTK mutations were detected and most commonly included gatekeeper mutations (T474I/F/S/L/Y, n=25 in 22 pts), kinase-impaired (L528W, n=14 in 14 pts), C481S/R/Y (n=6 in 4 pts) and others proximal to the ATP-binding pocket (n=7 in 5 pts), including D539A/G/H (n=3 in 1 pt), V416L (n=2 in 2 pts), Y545N (n=1) and A428D ([n=1] Figure). A total of 83 non-BTK acquired mutations were observed at PD in 45 pts (52%), including 14 TP53 mutations in 12 pts (14%), 6 PLCG2 mutations in 6 pts (7%), 6 PIK3CA mutations in 6 pts (7%) and 3 BCL2 mutations in 3 pts (3%).
Conclusions: Despite this cohort representing the first relapsing CLL patients from BRUIN and presenting with frequent baseline BTK mutations, response to pirtobrutinib was high, with an ORR of 83%, and substantial clearance of BTK C481 clones. At progression, the majority of pts (56%) either acquired non-BTK mutations or did not acquire any resistance mutations in this targeted panel, suggesting alternative resistance mechanisms. A smaller group of patients (44%) displayed emergence of non-C481 clones, particularly gatekeeper T474 and kinase-impaired L528W mutations. Whether similar patterns of resistance would manifest if pirtobrutinib was utilized in earlier lines of therapy or prior to cBTKi treatment remains uncertain.
OffLabel Disclosure:
Brown:Grifols Worldwide Operations: Consultancy; Pfizer: Consultancy; Merck: Consultancy; Genentech/Roche: Consultancy; Kite: Consultancy; iOnctura: Consultancy, Research Funding; Gilead: Research Funding; Loxo/Lilly: Consultancy, Research Funding; Pharmacyclics: Consultancy; SecuraBio: Research Funding; BeiGene: Consultancy, Research Funding; Alloplex Biotherapeutics: Consultancy; Acerta/AstraZeneca: Consultancy; Numab Therapeutics: Consultancy; Hutchmed: Consultancy; Abbvie: Consultancy; TG Therapeutics: Research Funding; MEI Pharma: Research Funding. Nguyen:Loxo@Lilly: Current Employment. Won:Loxo@Lilly: Current Employment; Eli Lilly and Company: Current equity holder in publicly-traded company. McNeely:Loxo@Lilly: Current Employment; Eli Lilly and Company: Current equity holder in publicly-traded company. Marella:Loxo@Lilly: Current Employment; Eli Lilly and Company: Current equity holder in publicly-traded company. Ebata:Loxo@Lilly: Current Employment; Eli Lilly and Company: Current equity holder in publicly-traded company. Woyach:Newave: Consultancy; Loxo: Consultancy; Beigene: Consultancy; AstraZeneca: Consultancy; Abbvie: Consultancy; Schrodinger: Research Funding; Morphosys: Research Funding; Karyopharm: Research Funding; Janssen: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding. Patel:Xencor: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; Fate Therapeutics: Research Funding; Epizyme: Consultancy, Research Funding; ADC Therapeutics: Consultancy; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; Kite: Consultancy, Research Funding, Speakers Bureau; Loxo Oncology: Consultancy, Research Funding; Merck: Consultancy, Research Funding; MEI Pharma: Consultancy, Research Funding; Morphosys: Consultancy; Nurix: Research Funding; Pharmacyclics/Janssen: Consultancy, Research Funding; Trillium Therapeutics/Pfizer: Consultancy, Research Funding; TG Therapeutics: Consultancy, Speakers Bureau; Sunesis Pharmaceuticals: Research Funding; CRISPR Therapeutics: Research Funding; Caribou Biosciences: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; BeiGene: Consultancy; Curis, Inc: Research Funding; Adaptive Biotechnologies: Research Funding; Abbvie: Consultancy. Tam:Janssen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; BeiGene: Honoraria, Research Funding; LOXO: Honoraria; Novartis: Honoraria; Roche: Honoraria. Eyre:Roche: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Loxo@Lilly: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy; Autolus: Consultancy; Beigene: Consultancy, Honoraria, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; KITE Gilead: Consultancy, Honoraria, Speakers Bureau. Cheah:Lilly: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; TG therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZenecca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Ascentage Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Research Funding; Menarini: Consultancy, Honoraria; Genmab: Consultancy, Honoraria; Dizal: Consultancy, Honoraria. Shah:Tundra Therapeutics: Current holder of stock options in a privately-held company; BMS/Juno: Consultancy; Epizyme: Consultancy; TG therapeutic: Consultancy; Novartis: Consultancy; Janssen: Consultancy; LOXO-Lilly: Consultancy, Other: Travel support; Umoja: Consultancy; Seattle Genetics: Consultancy; Gilead/Kite: Consultancy; Incyte: Consultancy; Abbvie: Consultancy; Lilly Oncology: Consultancy, Research Funding; Miltenyi Biotec: Consultancy, Other: Travel support, Research Funding. Ghia:BeiGene: Consultancy, Honoraria, Research Funding; MSD: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Lilly/Loxo Oncology: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding. Jurczak:AstraZeneca: Consultancy; BeiGene: Consultancy; AbbVie: Consultancy; Eli Lilly: Consultancy; Pfizer: Consultancy; Roche: Consultancy; SOBI: Consultancy; Takeda: Consultancy; AbbVie: Research Funding; AstraZeneca: Research Funding; Bayer: Research Funding; BeiGene: Research Funding; Celgene: Research Funding; Janssen: Research Funding; Eli Lilly: Research Funding; Merck: Research Funding; Pfizer: Research Funding; Roche: Research Funding; SOBI: Research Funding; Takeda: Research Funding. Balbas:Eli Lilly and Company: Current Employment, Current equity holder in publicly-traded company. Nair:Loxo@Lilly: Current Employment. Abada:Eli Lilly and Company: Current Employment, Current equity holder in publicly-traded company. Wang:Eli Lilly and Company: Current Employment, Current equity holder in publicly-traded company. Wang:Loxo@Lilly: Current Employment. Roeker:Curio: Other: CME speaker; Pharmacyclics: Consultancy; Pfizer: Consultancy, Research Funding; DAVA: Other: CME speaker; Genentech: Research Funding; TG Therapeutics: Consultancy; AbbVie: Consultancy, Research Funding; Adaptive Biotechnologies: Research Funding; Abbott Laboratories: Current equity holder in publicly-traded company; Ascentage: Consultancy; PeerView: Other: CME speaker; Janssen: Consultancy; AstraZeneca: Consultancy, Research Funding; Medscape: Other: CME speaker; Loxo Oncology: Consultancy, Other: travel support, Research Funding; Beigene: Consultancy; Aptose Biosciences: Research Funding; Dren Bio: Research Funding; Qilu Puget Sound Biotherapeutics: Research Funding. Gandhi:Sunesis: Honoraria, Research Funding; AbbVie: Research Funding; Clear Creek Bio: Consultancy, Research Funding; LOXO: Research Funding; Dava Oncology: Honoraria; Pharmacyclics: Research Funding. Wierda:Miragen: Research Funding; Sunesis: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Bristol Myers Squibb (Juno & Celgene): Consultancy, Research Funding; Numab THerapeutics: Research Funding; Pharmacyclics LLC: Research Funding; Gilead Sciences: Research Funding; Loxo Oncology, Inc./Lilly: Research Funding; Accutar Biotechnology: Research Funding; National Comprehensive Cancer Network: Other: Nonrelevant Financial Relationship/Chair, CLL). Supported by the NIH/NCI under award number P30 CA016672 and used MDACC Cancer Center Support Grant (CCSG) shared resources; NIH P30 CA016672/MDACC Cancer Center Support Grant: Research Funding; AstraZeneca/Acerta Pharma: Consultancy, Research Funding; KITE Pharma: Research Funding; GlaxoSmithKline: Research Funding; Genentech: Research Funding; Janssens Biotech: Research Funding; Cyclacel: Consultancy, Research Funding; Janssens Biotech Inc: Research Funding; AbbVie: Consultancy, Research Funding; Juno Therapeutics: Research Funding; Nurix THerapeutics: Research Funding; GSK/Novartis: Research Funding.
Pirtobrutinib is approved in the USA to treat R/R mantle cell lymphoma after at least two lines of systemic therapy including prior BTKi treatment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal