Background: In response to the ever-evolving challenges posed by the antigenically diverse Omicron variants, the deployment of bivalent mRNA booster vaccines encompassing both the ancestral (WA1) and Omicron BA.5 spike components was initiated in fall of 2022. The real-world impact of these bivalent vaccines is largely unknown in patients with Multiple Myeloma (MM). We characterize the humoral and cellular immune responses in patients with MM before and after receiving the bivalent booster implementing cutting edge methodologies to identify patterns associated with continuing vulnerability in the current post-pandemic era.
Methods: We studied the humoral and cellular immune responses before and after bivalent booster immunization in 48 MM patients. Spike binding IgG antibody levels were measured by SARS-CoV-2 spike binding ELISA and neutralization capacity was assessed by a SARS-CoV-2 multi-cycle microneutralization assay. We measured spike specific T cell function using the QuantiFERON SARS-CoV-2 (Qiagen) assay as well as flow cytometry-based T cell assays quantifying interferon-gamma (IFNg) production. In a subset of 38 patients, immune profiling was performed using high-dimensional flow cytometry. The frequencies of dendritic cells (DCs), B cells, Natural Killer (NK) cells and T follicular helper cells (TFH) were compared between responders and non-responders.
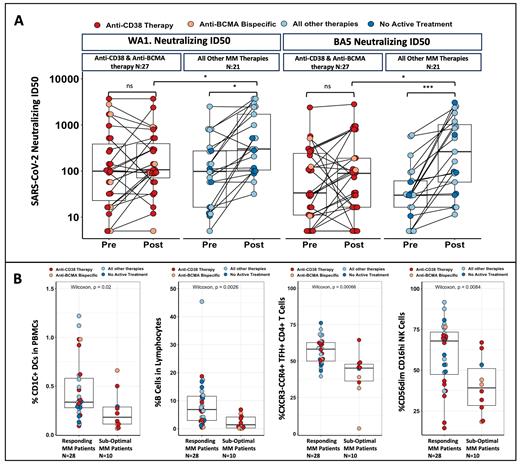
Results: The serological effect of the bivalent vaccination in MM patients is nuanced with the overall cohort not demonstrating a significant increase post bivalent vaccination (median 196 AU/mL prior to bivalent to median 276 post bivalent p=0.11). The lack of antibody response was driven by patients undergoing anti-CD38 and anti-BCMA antibody therapy as compared to MM patients undergoing other therapies who demonstrated a significant increase in antibody levels post vaccination (p=0.021). Patients on anti-CD38 and anti-BCMA antibody therapy also did not significantly increase neutralizing capacity to WA1 (p=0.42) or BA.5 omicron strain (p=0.48) while MM patients on other therapies benefitted significantly, as illustrated by the increased neutralizing capacity of WA1 (p=0.024) and BA.5 (p=0.0055 Figure 1A). In addition to quantifying antibody responses, we profiled T cell responses using our published flow cytometry method using WA1 anti-S peptides as well as the QuantiFERON SARS-CoV-2 RUO assay (Qiagen). Spike reactive T cell responses significantly correlated with anti-Spike IgG levels in MM patients (r 2=0.49, p<0.001), WA1 neutralizing ID50 (R 2 = 0.6, p<0.01) and BA.5 neutralizing ID50 (R 2= 0.54, p=0.01) reinforcing the fact that T cell compensation is lacking in MM patients. We separated the lowest quantile (patients with Anti-S IgG <156 AU/mL) to delineate suboptimal producing patients compared to the remaining MM anti-S producing patients. Non-responders exhibited deficiencies in immune populations involved in robust antibody production including lower frequency of CD1c+ DCs (p=0.02 Figure 1B), B Cells (p<0.01 Figure 1B) and circulating CXCR3-CCR4+TFH cells (p<0.01 Figure 1B). Furthermore, immature NK cells were the predominant population in non-responders, while responders had more cytotoxic mature NK phenotype (p<0.01 Figure 1B) after bivalent vaccination.
Conclusions: Our study highlights the varying immune responses observed in MM patients after receiving bivalent COVID-19 vaccination. Specifically, a subgroup of MM patients undergoing anti-CD38 and anti-BCMA therapy experience impairments in immune cells such DCs, B cells, NK cells and TFH cells, leading to an inability to generate adequate antibody and T cell responses to vaccinations. Our results indicate that the QuantiFERON SARS-CoV-2 assay could be deployed for clinical use bridging the need for suitable laboratory tests to measure SARS-CoV-2 specific T cell responses. Ongoing research will further investigate whether the compromised immune machinery and diminished T cell activity identified in this study can serve as discriminators for identifying MM patients at high risk of infectious complications stemming from anti-BCMA therapies. Such insights will aid in developing targeted strategies to address these challenges and improve patient outcomes.
Disclosures
Cordon-Cardo:Kantaro: Patents & Royalties. Krammer:Kantaro: Patents & Royalties. Jagannath:Janssen Pharmaceuticals: Consultancy, Honoraria; Regeneron: Consultancy; BMS: Consultancy, Honoraria; IMS: Membership on an entity's Board of Directors or advisory committees; Genmab: Other: DMC chairman; ASH: Membership on an entity's Board of Directors or advisory committees; Caribou: Consultancy; Sanofi: Consultancy, Other: DMC Chariman; Takeda: Consultancy; Karyopharma: Consultancy; SOHO: Membership on an entity's Board of Directors or advisory committees; Legend Biotech: Consultancy. Simon:Kantaro: Patents & Royalties: Serological Tests. Parekh:Karyopharm Therapeutics: Research Funding; Grail, LLC: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Celgene/BMS Corporation: Research Funding; Caribou Biosciences: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal