Background
In chronic lymphocytic leukemia (CLL) triple combinations of BTK inhibitors (BTKi), venetoclax (ven) and CD20 antibodies have been tested mostly as first-line treatments and so far sparse data is available on their efficacy in the relapsed/refractory (r/r) setting. In this phase 2 trial, we evaluate the combination of zanubrutinib (zanu), ven and obinutuzumab (obi) in r/r CLL. In order to tailor therapy to the different needs of differently pretreated patients (pts), treatment duration was based on detection of measurable residual disease (MRD). Furthermore, a ctDNA-based MRD monitoring and a ctDNA-based real-time screen of resistance and driver mutations were performed throughout the study.
Methods
The CLL2-BZAG study evaluated a combination of obi, zanu and ven after an optional debulking with bendamustine in r/r CLL. In the induction, obi was administered 3 times in cycle 1 (days 1/2, 8 & 15) and monthly in cycles 2-6. Zanu was added in cycle 2 and ven in cycle 3 with a dose ramp-up over 5 weeks. Maintenance with continuous zanu and ven and 3-monthly obi was administered either until achievement of a deep remission with undetectable MRD <10 -4 (uMRD) in peripheral blood (PB) or for up to 24 months.
The primary endpoint was the uMRD rate by flow cytometry (FCM) in PB at the final restaging (RE, after 6 months of triple therapy). MRD was measured by FCM from PB and by digital droplet PCR (ddPCR) of patient-specific VDJ rearrangements from blood plasma. uMRD was defined as <1 CLL cell/10,000 leukocytes (<10 -4) and no detectable patient-specific VDJ ctDNA.
Results
Between 11/2020 and 10/2022, 42 pts were enrolled, 2 pts with ≤2 induction cycles were excluded from the analysis population as predefined by the protocol. Median age was 64 years (range 40-82), 32.5% of pts were female. All 40 pts had r/r CLL with a median of 1 prior therapy (range 1-5), 18 pts (45%) had already received a BTKi, 7 pts ven (17.5%), of these 5 pts (12.5%) had received both. Fifteen pts (37.5%) had a del(17p)/ TP53 mutation, 31 (77.5%) had unmutated IGHV and 19 of 38 pts with available information (50%) a complex karyotype.
Nineteen pts (47.5%) received bendamustine debulking, 37 pts (92.5%) completed 6 induction cycles and 19 pts (47.5%) have already stopped MRD-guided maintenance treatment with 2 pts who discontinued maintenance early due to adverse events (AEs).
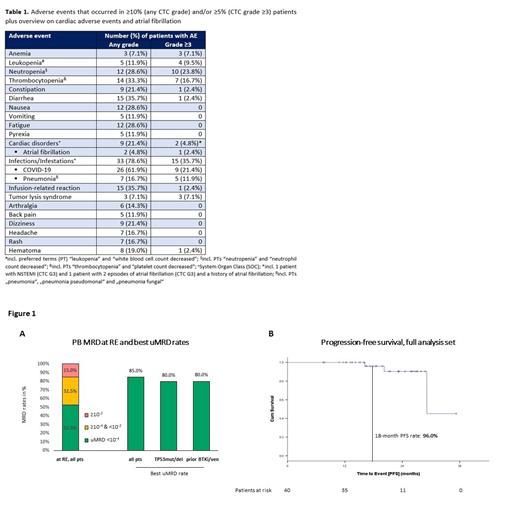
With a median observation time of 21.5 months (range 8.0-35.3), 445 AEs were reported, including 90 AEs of CTC grades 3-4. Two pts had fatal AEs (COVID-19, fungal pneumonia secondary to COVID-19). The most common AEs were COVID-19 (n=26 pts), diarrhea (n=15), infusion-related reactions (n=15), thrombocytopenia (n=14), nausea (n=12), fatigue (n=12) and neutropenia (n=12). The most frequent grade ≥3 AEs were neutropenia (n=10 pts), COVID-19 (n=9), thrombocytopenia (n=7) and pneumonia (n=5, Table 1). Two pts had grade ≥3 cardiac AEs (1 pt with grade 3 non-ST-elevation myocardial infarction, 1 pt with 2 AEs of grade 3 atrial fibrillation and a history of atrial fibrillation).
At RE all pts responded and 21 (52.5%) showed uMRD in PB ( Figure 1A). In many pts remissions deepened over time, with 34 (85%) achieving uMRD in PB in the course of the study. The proportion of pts with uMRD was similar in pts with TP53 aberrations (80%) and in pts previously exposed to venetoclax and/or BTKi (80%). The estimated PFS and OS rates at 18 months were 96% and 96.8% ( Figure 1B). PFS rates at 18 months were similar for pts with TP53 aberrations (100%) and pts pretreated with BTKi/ven (90%). Two pts had a disease progression per iwCLL criteria, however no pt has yet required a subsequent treatment. Of 22 pts who achieved uMRD and are now off-treatment, only 1 had MRD recurrence (≥10 -4) during follow-up.
Serial MRD (FCM) and ctDNA (ddPCR) assessments were done on 281 paired blood/plasma samples throughout the study. At RE, 8 pts who achieved uMRD by FCM still had detectable VDJ ctDNA in blood plasma. Four pts without ctDNA were still MRD positive by FCM at RE. Apart from one PLCG2_S707F mutation that occurred 11 months after the end of treatment, no acquisition of known resistance mutations - in particular in BTK, BCL2 - was observed using ddPCR.
Conclusions
The time-limited MRD-guided triple combination of zanu, ven and obi induced deep remissions in a r/r CLL population enriched for pts previously treated with BTKi and/or ven. Apart from COVID-19, low rates of infectious AEs were observed and cardiac toxicities were rare.
OffLabel Disclosure:
Furstenau:Abbvie: Honoraria, Research Funding; Roche: Research Funding; Janssen: Research Funding; AstraZeneca: Research Funding; BeiGene: Research Funding. Schneider:Abbvie: Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; BeiGene: Other: travel support; Jannsen Cilag: Consultancy. Tausch:BeiGene: Consultancy, Other: Travel support, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Other: travel support, Speakers Bureau; Janssen-Cilag: Consultancy, Honoraria, Other: travel support, Speakers Bureau; Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau; Abbvie: Consultancy, Honoraria, Other: Travel Support, Research Funding, Speakers Bureau. Ritgen:Janssen: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: travel support; Abbvie: Consultancy, Research Funding. Hebart:AbbVie, AstraZeneca, Celgene, Janssen, Roche, and Beigene: Honoraria. Illert:Amgen: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Graeven:Ipsen: Research Funding; Celltrion: Honoraria; GSK: Other: travel support; Novartis: Honoraria; Fujifilm: Honoraria; Sanofi: Honoraria; MSD: Consultancy, Honoraria; BMS: Honoraria; AstraZeneca: Honoraria; Amgen: Consultancy, Honoraria; Boehringer Ingelheim: Honoraria, Other: travel support; BioNTech: Current holder of stock options in a privately-held company; MacroGenics: Honoraria, Research Funding. Fink:Abbvie: Other: travel support; AstraZeneca: Consultancy, Honoraria, Research Funding. Simon:AstraZeneca: Research Funding; Lilly Pharma: Other: Travel support. Fischer:Roche: Honoraria, Other: Travel Support; AstraZeneca: Consultancy; Abbvie: Honoraria, Other: TRavel support. Al-Sawaf:Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eli Lilly: Speakers Bureau; BeiGene: Research Funding, Speakers Bureau; Adaptive: Speakers Bureau; Ascentage: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Langerbeins:Abbvie: Honoraria, Other: travel support; Janssen: Honoraria, Other: travel support, Research Funding; Beigene: Honoraria, Other: travel support; AstraZeneca: Honoraria, Other: travel support. Weiss:Qiagen: Current Employment. Kreuzer:Abbvie: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; Roche: Consultancy, Research Funding, Speakers Bureau. Brüggemann:Incyte: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Affimed: Research Funding; Regeneron: Research Funding; Janssen: Speakers Bureau; BD: Speakers Bureau; Pfizer: Speakers Bureau. Eichhorst:Abbvie: Consultancy, Honoraria, Research Funding, Speakers Bureau; BeiGene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead: Consultancy, Research Funding; F. Hoffmann-La Roche Ltd: Honoraria, Research Funding, Speakers Bureau; Lilly: Consultancy, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; MSD: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau. Stilgenbauer:Amgen: Consultancy, Honoraria, Other: travel support, Research Funding; Abbvie: Consultancy, Honoraria, Other: travel support, Research Funding; Celgene: Consultancy, Honoraria, Other: travel support, Research Funding; Gilead: Consultancy, Honoraria, Other: travel support, Research Funding; GSK: Consultancy, Honoraria, Other: travel support, Research Funding; Roche: Consultancy, Honoraria, Other: travel support, Research Funding; Janssen: Consultancy, Honoraria, Other: travel support, Research Funding; Novartis: Consultancy, Honoraria, Other: travel support, Research Funding; Sunesis: Consultancy, Honoraria, Other: travel support, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: travel support, Research Funding. Hallek:Abbvie: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding. Cramer:Novartis: Research Funding; Gilead: Research Funding; BeiGene: Consultancy, Other: travel support, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: travel support, Research Funding; Janssen: Honoraria, Research Funding; Acerta: Research Funding; Roche: Honoraria, Other: travel support, Research Funding; Abbvie: Consultancy, Honoraria, Other: travel support, Research Funding; BMS: Honoraria.
Zanubrutinib, Venetoclax, Obinutuzumab is a combination that is not approved for the treatment of CLL

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal