Page 693: The symbol key in Figure 1A, which applies to Figure 1B-E, G-H, and J, is incorrect. The corrected Figure 1 is shown below.
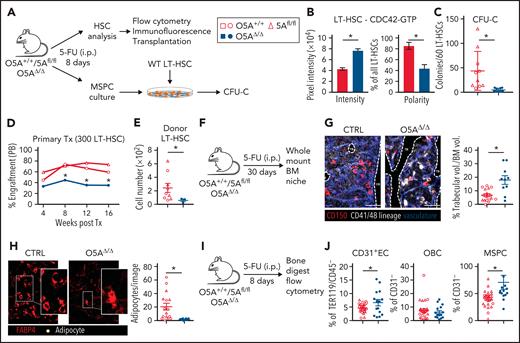
Stress-induced niche remodeling in O5AΔ/Δ mice. (A) Experimental design for IP injection of 5-Fluorouracil (5-FU) in coculture assay of MSPCs and LT-HSC. The following genotypes were analyzed by flow cytometry and IF 8 days after treatment: Control (CTRL): O5A+/+, n = 5, 5Afl/fl, n = 7 and O5AΔ/Δ, n = 10. (B) Protein content and polarity of LT-HSCs stained for CDC42-GTP (n = 30), measured by ImageJ software. (C) Number of colonies from 60 LT-HSCs after coculture for 6 days on MSPCs isolated from 5-FU injected mice with the following genotype: (CTRL): O5A+/+, n = 5, 5Afl/fl, n = 5 and O5AΔ/Δ, n = 10. (D) Primary transplantation (Tx) of 300 sorted LT-HSCs 8 days after 5-FU injection into lethally irradiated 129∗Ly5.1 WT recipients. Experimental groups: CTRL: O5A+/+, n = 5 and 5Afl/fl, n = 4 and O5AΔ/Δ, n = 7. Graph showing the donor engraftment in PB. (E) Absolute numbers of donor-type LT-HSCs in the BM, 16 weeks after Tx. For flow cytometry gating strategy, see supplemental Figure 1. (F) Experimental design for IP injection of 5-FU in 8- to 10-week-old mice of following genotypes: CTRL: O5A+/+ n = 2, 5Afl/fl n = 2 and O5AΔ/Δ, n = 2. BM analysis 30 days after treatment. (G) Stacked whole-mount images from epiphyseal/metaphyseal BM. FABP4+ vasculature is shown in blue. CD150 is shown in red, other hematopoietic markers (CD41, CD48, and lineage) are shown in gray. The dashed lines denote the endosteum. Scale bar, 100 μm; n = 10 images (n = 6 images 5Afl/fl PBS) from 2 mice each group. The results represent 2 independent experiments. The graph showing the % of trabecular volume per BM volume. (H) Stacked whole-mount images from epiphyseal/metaphyseal BM. FABP4+ adipocytes are shown in red. Adipocytes are additionally marked by a yellow circle. Extended focus projection images. Scale bar, 100 μm; n = 10 images from 2 mice each group, femora. The graph on the right shows the number of adipocytes per image. (I) Experimental design for analysis of bone-digested stromal cells, isolated from compact bones, 8 days post 5-FU treatment. (J) Relative numbers of CD31+ ECs, OBCs, and immature MSPCs; flow cytometry gating strategy in supplemental Figure 1. ∗P < .05 (nonparametric Mann-Whitney test: B-G,J). The results represent 2 to 3 independent experiments. Data are represented as dots per mouse or cell and the mean ± SEM. Symbol legends as shown in Figure 1A.
Stress-induced niche remodeling in O5AΔ/Δ mice. (A) Experimental design for IP injection of 5-Fluorouracil (5-FU) in coculture assay of MSPCs and LT-HSC. The following genotypes were analyzed by flow cytometry and IF 8 days after treatment: Control (CTRL): O5A+/+, n = 5, 5Afl/fl, n = 7 and O5AΔ/Δ, n = 10. (B) Protein content and polarity of LT-HSCs stained for CDC42-GTP (n = 30), measured by ImageJ software. (C) Number of colonies from 60 LT-HSCs after coculture for 6 days on MSPCs isolated from 5-FU injected mice with the following genotype: (CTRL): O5A+/+, n = 5, 5Afl/fl, n = 5 and O5AΔ/Δ, n = 10. (D) Primary transplantation (Tx) of 300 sorted LT-HSCs 8 days after 5-FU injection into lethally irradiated 129∗Ly5.1 WT recipients. Experimental groups: CTRL: O5A+/+, n = 5 and 5Afl/fl, n = 4 and O5AΔ/Δ, n = 7. Graph showing the donor engraftment in PB. (E) Absolute numbers of donor-type LT-HSCs in the BM, 16 weeks after Tx. For flow cytometry gating strategy, see supplemental Figure 1. (F) Experimental design for IP injection of 5-FU in 8- to 10-week-old mice of following genotypes: CTRL: O5A+/+ n = 2, 5Afl/fl n = 2 and O5AΔ/Δ, n = 2. BM analysis 30 days after treatment. (G) Stacked whole-mount images from epiphyseal/metaphyseal BM. FABP4+ vasculature is shown in blue. CD150 is shown in red, other hematopoietic markers (CD41, CD48, and lineage) are shown in gray. The dashed lines denote the endosteum. Scale bar, 100 μm; n = 10 images (n = 6 images 5Afl/fl PBS) from 2 mice each group. The results represent 2 independent experiments. The graph showing the % of trabecular volume per BM volume. (H) Stacked whole-mount images from epiphyseal/metaphyseal BM. FABP4+ adipocytes are shown in red. Adipocytes are additionally marked by a yellow circle. Extended focus projection images. Scale bar, 100 μm; n = 10 images from 2 mice each group, femora. The graph on the right shows the number of adipocytes per image. (I) Experimental design for analysis of bone-digested stromal cells, isolated from compact bones, 8 days post 5-FU treatment. (J) Relative numbers of CD31+ ECs, OBCs, and immature MSPCs; flow cytometry gating strategy in supplemental Figure 1. ∗P < .05 (nonparametric Mann-Whitney test: B-G,J). The results represent 2 to 3 independent experiments. Data are represented as dots per mouse or cell and the mean ± SEM. Symbol legends as shown in Figure 1A.
Page 699: The symbol key in Figure 6A, which applies to Figure 6B, C, E, G, and I, is incorrect. In Figure 6B, the red symbols should be outlined in red rather than solid red. The corrected Figure 6 is shown below.
Attenuation of elevated CDC42 activity ensures survival. (A) Serial IP injection of 5-FU in the following genotypes: CTRL: O5A+/+ n = 6, 5Afl/fl n = 7 and O5AΔ/Δ n = 7 at day 0 and day 8. Additional in vivo IP injection of CDC42-GTP inhibitor CASIN at day 5 through day 8. Analysis of animal survival, hematopoiesis (BM from 4 flushed long bones), and compact bone-derived MSPCs at day 14. (B) Percentage of mice survival after serial 5-FU treatment. Graph shows the survival curve of CTRL and O5AΔ/Δ mice treated with CASIN (+) or vehicle (−). (C) Graph shows the total BM cell number (left) and relative number of LSK cells (middle) and MPs (right) at day 14 of CTRL mice with vehicle ([−]; O5A+/+ n = 6, 5Afl/fl n = 7), O5AΔ/Δ mice with vehicle ([−]; n = 5, mice analyzed shortly before death) and O5AΔ/Δ mice with CASIN ([+]; n = 5). (D,F,H,J) Representative contour plots from BM populations. Graphs show relative number of LT-HSCs and ST-HSCs (E-F), T cells and B cells (G-H), and Gr1+ myeloid cells (I-J). ∗P < .05 (nonparametric Mann-Whitney test: C,E,G,I). The results represent 2 to 3 independent experiments. Data are represented as mean ± SEM. Symbol legend shown in Figure 6A.
Attenuation of elevated CDC42 activity ensures survival. (A) Serial IP injection of 5-FU in the following genotypes: CTRL: O5A+/+ n = 6, 5Afl/fl n = 7 and O5AΔ/Δ n = 7 at day 0 and day 8. Additional in vivo IP injection of CDC42-GTP inhibitor CASIN at day 5 through day 8. Analysis of animal survival, hematopoiesis (BM from 4 flushed long bones), and compact bone-derived MSPCs at day 14. (B) Percentage of mice survival after serial 5-FU treatment. Graph shows the survival curve of CTRL and O5AΔ/Δ mice treated with CASIN (+) or vehicle (−). (C) Graph shows the total BM cell number (left) and relative number of LSK cells (middle) and MPs (right) at day 14 of CTRL mice with vehicle ([−]; O5A+/+ n = 6, 5Afl/fl n = 7), O5AΔ/Δ mice with vehicle ([−]; n = 5, mice analyzed shortly before death) and O5AΔ/Δ mice with CASIN ([+]; n = 5). (D,F,H,J) Representative contour plots from BM populations. Graphs show relative number of LT-HSCs and ST-HSCs (E-F), T cells and B cells (G-H), and Gr1+ myeloid cells (I-J). ∗P < .05 (nonparametric Mann-Whitney test: C,E,G,I). The results represent 2 to 3 independent experiments. Data are represented as mean ± SEM. Symbol legend shown in Figure 6A.
Page 701: The symbols in the graphs in Figure 7B-G do not match the symbol key in Figure 7A. The corrected Figure 7 is shown below.
Attenuation of elevated CDC42 activity during auto/mitophagy. (A) IP injection of 5-FU in O5AΔ/Δ mice at day 0. Additional in vivo IP injection of vehicle ([−], n = 5) or CASIN ([+], n = 4) at day 5 through day 8. Rescue and analysis of autophagy relevant mechanisms in compact bone-derived MSPCs, isolated at day 8 and cultured until passage 4. (A-G) Shown are the results of O5AΔ/Δ mice with vehicle (−) and CASIN (+) treatment. CASIN-treated mice show the same phenotype as the control groups 5Afl/fl and O5A+/+ (Figure 3). (B) Fluorescent microscopy images of LC3 (red) and DAPI (blue) staining. Graph shows the pixel intensity as measured by ImageJ software. (C) Fluorescent microscopy image of CDC42-GTP (green), LC3 (red), and DAPI (blue) staining. The graph shows the percentage of MSPCs with colocalization measured by ImageJ software (plugin colocalization) and visualized in white. (D) Fluorescent microscopy images of F-actin (phalloidin/green) and LC3 (red) in MSPCs. Colocalization was measured by ImageJ software (plug-in colocalization) and visualized in white. The graph shows colocalization counted with ImageJ software. (E) Fluorescent microscopy images of LAMP1 (green) and DAPI (blue) staining. The pixel intensity (left graph) and Feret’s diameter (right graph) were measured by ImageJ software. (F) Fluorescent microscopy images of LAMP1 (green), LC3 (red), and DAPI (blue) staining. Yellow arrows show colocalized vesicle staining, red arrows depict LC3+ vesicles that did not colocalize with green LAMP1+ vesicles. Perinuclear colocalization of LAMP1 and LC3 measured by ImageJ software (plugin colocalization, depicted in white). (G) Representative FACS plots and quantification (graph) of Cyto-ID dye levels (DMFI: Cyto-ID dye level chloroquine treated, Cyto-ID dye level w/o treatment). (H) Fluorescent microscopy images of TOMM20 (green), LC3 (red), and DAPI (blue) staining. Colocalization was measured by ImageJ software (plugin colocalization) and visualized in the bottom row in white. Scale bars, 10 μm. ∗P < .05 (2-sided parametric Student’s t test; B-H). The results represent 2 independent experiments. Data are represented as mean ± SEM. Symbol legend shown in Figure 7A.
Attenuation of elevated CDC42 activity during auto/mitophagy. (A) IP injection of 5-FU in O5AΔ/Δ mice at day 0. Additional in vivo IP injection of vehicle ([−], n = 5) or CASIN ([+], n = 4) at day 5 through day 8. Rescue and analysis of autophagy relevant mechanisms in compact bone-derived MSPCs, isolated at day 8 and cultured until passage 4. (A-G) Shown are the results of O5AΔ/Δ mice with vehicle (−) and CASIN (+) treatment. CASIN-treated mice show the same phenotype as the control groups 5Afl/fl and O5A+/+ (Figure 3). (B) Fluorescent microscopy images of LC3 (red) and DAPI (blue) staining. Graph shows the pixel intensity as measured by ImageJ software. (C) Fluorescent microscopy image of CDC42-GTP (green), LC3 (red), and DAPI (blue) staining. The graph shows the percentage of MSPCs with colocalization measured by ImageJ software (plugin colocalization) and visualized in white. (D) Fluorescent microscopy images of F-actin (phalloidin/green) and LC3 (red) in MSPCs. Colocalization was measured by ImageJ software (plug-in colocalization) and visualized in white. The graph shows colocalization counted with ImageJ software. (E) Fluorescent microscopy images of LAMP1 (green) and DAPI (blue) staining. The pixel intensity (left graph) and Feret’s diameter (right graph) were measured by ImageJ software. (F) Fluorescent microscopy images of LAMP1 (green), LC3 (red), and DAPI (blue) staining. Yellow arrows show colocalized vesicle staining, red arrows depict LC3+ vesicles that did not colocalize with green LAMP1+ vesicles. Perinuclear colocalization of LAMP1 and LC3 measured by ImageJ software (plugin colocalization, depicted in white). (G) Representative FACS plots and quantification (graph) of Cyto-ID dye levels (DMFI: Cyto-ID dye level chloroquine treated, Cyto-ID dye level w/o treatment). (H) Fluorescent microscopy images of TOMM20 (green), LC3 (red), and DAPI (blue) staining. Colocalization was measured by ImageJ software (plugin colocalization) and visualized in the bottom row in white. Scale bars, 10 μm. ∗P < .05 (2-sided parametric Student’s t test; B-H). The results represent 2 independent experiments. Data are represented as mean ± SEM. Symbol legend shown in Figure 7A.
The publisher apologizes for the errors, which occurred during the publication process and (except for the red symbols in Figure 6B) have been corrected in the online version of the article.

![Attenuation of elevated CDC42 activity ensures survival. (A) Serial IP injection of 5-FU in the following genotypes: CTRL: O5A+/+ n = 6, 5Afl/fl n = 7 and O5AΔ/Δ n = 7 at day 0 and day 8. Additional in vivo IP injection of CDC42-GTP inhibitor CASIN at day 5 through day 8. Analysis of animal survival, hematopoiesis (BM from 4 flushed long bones), and compact bone-derived MSPCs at day 14. (B) Percentage of mice survival after serial 5-FU treatment. Graph shows the survival curve of CTRL and O5AΔ/Δ mice treated with CASIN (+) or vehicle (−). (C) Graph shows the total BM cell number (left) and relative number of LSK cells (middle) and MPs (right) at day 14 of CTRL mice with vehicle ([−]; O5A+/+ n = 6, 5Afl/fl n = 7), O5AΔ/Δ mice with vehicle ([−]; n = 5, mice analyzed shortly before death) and O5AΔ/Δ mice with CASIN ([+]; n = 5). (D,F,H,J) Representative contour plots from BM populations. Graphs show relative number of LT-HSCs and ST-HSCs (E-F), T cells and B cells (G-H), and Gr1+ myeloid cells (I-J). ∗P < .05 (nonparametric Mann-Whitney test: C,E,G,I). The results represent 2 to 3 independent experiments. Data are represented as mean ± SEM. Symbol legend shown in Figure 6A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/8/10.1182_blood.2023021108/2/m_blood_bld-2023-021108-gr2.jpeg?Expires=1769382771&Signature=WKfPs9HEZ92o1dYJIZ7E1j-pqOGOvnrOiXcFfvM0jdkpFnTPFAG4r5kwm-JbLkttbveZ6rdPinsRNMX1-S6d5tQ4TbW9cfLN0HLNXcjuBj81hGwzcKQoixAB~9~z-rbcBlbeTJDTqDboYQTuin0lz7Yw~~miLpXgNW1zn45Ms~IJnNkVyH0NO4N~B1axFlXBOWLaT8g60dzz0APbJAmyXSUT1g02CzSn048o~hiFjas9pDmabxNSQuGdncxRdzCZokrmupEqKzQ2rgn4CFgvLNt0ZPW9advxhxQ1Jq3nQJa2N3Fbx1rDTTU-ir9eKtfKnL6q8dwhw5xjJsQ813v2ag__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Attenuation of elevated CDC42 activity during auto/mitophagy. (A) IP injection of 5-FU in O5AΔ/Δ mice at day 0. Additional in vivo IP injection of vehicle ([−], n = 5) or CASIN ([+], n = 4) at day 5 through day 8. Rescue and analysis of autophagy relevant mechanisms in compact bone-derived MSPCs, isolated at day 8 and cultured until passage 4. (A-G) Shown are the results of O5AΔ/Δ mice with vehicle (−) and CASIN (+) treatment. CASIN-treated mice show the same phenotype as the control groups 5Afl/fl and O5A+/+ (Figure 3). (B) Fluorescent microscopy images of LC3 (red) and DAPI (blue) staining. Graph shows the pixel intensity as measured by ImageJ software. (C) Fluorescent microscopy image of CDC42-GTP (green), LC3 (red), and DAPI (blue) staining. The graph shows the percentage of MSPCs with colocalization measured by ImageJ software (plugin colocalization) and visualized in white. (D) Fluorescent microscopy images of F-actin (phalloidin/green) and LC3 (red) in MSPCs. Colocalization was measured by ImageJ software (plug-in colocalization) and visualized in white. The graph shows colocalization counted with ImageJ software. (E) Fluorescent microscopy images of LAMP1 (green) and DAPI (blue) staining. The pixel intensity (left graph) and Feret’s diameter (right graph) were measured by ImageJ software. (F) Fluorescent microscopy images of LAMP1 (green), LC3 (red), and DAPI (blue) staining. Yellow arrows show colocalized vesicle staining, red arrows depict LC3+ vesicles that did not colocalize with green LAMP1+ vesicles. Perinuclear colocalization of LAMP1 and LC3 measured by ImageJ software (plugin colocalization, depicted in white). (G) Representative FACS plots and quantification (graph) of Cyto-ID dye levels (DMFI: Cyto-ID dye level chloroquine treated, Cyto-ID dye level w/o treatment). (H) Fluorescent microscopy images of TOMM20 (green), LC3 (red), and DAPI (blue) staining. Colocalization was measured by ImageJ software (plugin colocalization) and visualized in the bottom row in white. Scale bars, 10 μm. ∗P < .05 (2-sided parametric Student’s t test; B-H). The results represent 2 independent experiments. Data are represented as mean ± SEM. Symbol legend shown in Figure 7A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/8/10.1182_blood.2023021108/2/m_blood_bld-2023-021108-gr3.jpeg?Expires=1769382771&Signature=tIT3gCkKkd32ixVnXfzMFdrcyp~Nv9oZVkuI1ZstcISW1tY97N3c4LHKLI0V4DDVNPhTUF0lMoshrmgSBtdQyWe3ilfKmKOhbfV9SgFibxVwYRBF-CqMYJetwgrxVVIUTV2EzjflTpTdkBgj7OUioHw-yxRcuY0IVng8nKtOW~HpIq3EuGGsY3lkNOaILpbmzznjkvrwSY~bBsgz5YjkeOEcedTNidNNvAvtPS0~i~-ACcnwQiCohQRHkEOTg2weCW1rOKchL3GALSAVEF2Mf~4N3hV-P~gdRcWiQDiRwenmF9OM3~RS5nplzLJUIk3xYLK44uyiBLycu3sQTbux7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal