Key Points
In early-stage favorable NLPHL, consolidation RT appears necessary to achieve the optimal disease control irrespective of the iPET result.
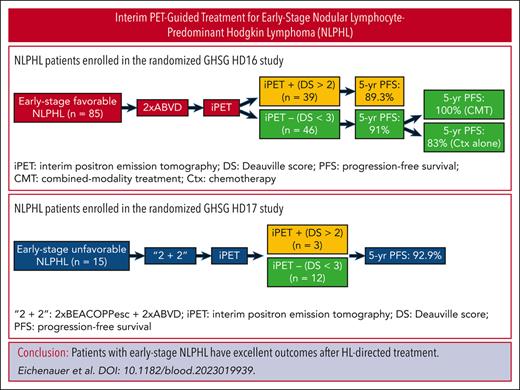
In early-stage NLPHL, Hodgkin lymphoma–directed approaches result in a 5-year PFS >90% and a 5-year overall survival of 100%.
Abstract
The optimal first-line treatment for nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) diagnosed in early stages is largely undefined. We, therefore, analyzed 100 NLPHL patients treated in the randomized HD16 (early-stage favorable; n = 85) and HD17 (early-stage unfavorable; n = 15) studies. These studies investigated the omission of consolidation radiotherapy (RT) in patients with a negative interim positron emission tomography (iPET) (ie, Deauville score <3) after chemotherapy (HD16: 2× doxorubicin, bleomycin, vinblastine, and dacarbazine [ABVD]; HD17: 2× escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone [BEACOPP] plus 2× ABVD). Patients with NLPHL treated in the HD16 and HD17 studies had 5-year progression-free survival (PFS) rates of 90.3% and 92.9%, respectively. Thus, the 5-year PFS did not differ significantly from that of patients with classical Hodgkin lymphoma treated within the same studies (HD16: P = .88; HD17: P = .50). Patients with early-stage favorable NLPHL who had a negative iPET after 2× ABVD and did not undergo consolidation RT tended to have a worse 5-year PFS than patients with a negative iPET who received consolidation RT (83% vs 100%; P = .05). There were 10 cases of NLPHL recurrence. However, no NLPHL patient died during follow-up. Hence, the 5-year overall survival rate was 100%. Taken together, contemporary Hodgkin lymphoma-directed treatment approaches result in excellent outcomes for patients with newly diagnosed early-stage NLPHL and, thus, represent valid treatment options. In early-stage favorable NLPHL, consolidation RT appears necessary after 2× ABVD to achieve the optimal disease control irrespective of the iPET result.
Introduction
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) is a rare disease, accounting for ∼5% of all Hodgkin lymphoma (HL) cases; pathological and clinical characteristics differ from those of classical HL (cHL). The malignant cells in NLPHL are consistently positive for CD20 but negative for CD30. The majority of patients with NLPHL initially present with early-stage disease.1 The clinical course of NLPHL is mostly indolent, resulting in a low excess mortality in comparison with that of the general population.2 However, ∼5% to 10% of patients experience histological transformation into aggressive B-cell non-Hodgkin lymphoma (B-NHL) within 10 years from the initial NLPHL diagnosis.3,4 NLPHL thus resembles low-grade B-NHL in some aspects, which has prompted the clinical advisory committee to propose nodular lymphocyte-predominant B-cell lymphoma as an alternative name.5
At most institutions, limited-field radiotherapy (RT) alone represents the standard treatment for patients with stage IA NLPHL without clinical risk factors.6,7 In contrast, there is no accepted standard of care for early-stage NLPHL other than that for stage IA disease without clinical risk factors.8 Patients mostly receive HL-directed approaches consisting of a brief chemotherapy followed by consolidation RT.9 However, B-NHL–directed protocols such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and rituximab, cyclophosphamide, vincristine, and prednisone (R-CVP) are also applied.10-13 Unlike in cHL, the role of treatment stratification based on interim positron emission tomography (iPET) is ill defined for patients presenting with NLPHL in early stages.14
To shed more light on the characteristics and outcomes of patients with early-stage NLPHL who had contemporary HL-directed treatment and to elucidate the role of iPET in these patients, we conducted a subgroup analysis of the randomized German Hodgkin Study Group (GHSG) HD16 and HD17 studies for individuals with the initial diagnosis of early-stage favorable and early-stage unfavorable HL, respectively.15-17
Patients and methods
Patients and treatment
Patients with newly diagnosed NLPHL (as confirmed via expert review) treated in the randomized GHSG studies HD16 for early favorable stages (stage I/II without clinical risk factors) and HD17 for early unfavorable stages (stage I/IIA with at least 1 of the following risk factors: large mediastinal mass, extranodal disease, elevated erythrocyte sedimentation rate, and involvement of ≥3 nodal areas or stage IIB with 1 or both of the following risk factors: elevated erythrocyte sedimentation rate and involvement of ≥3 nodal areas) were eligible for the present analysis. Patients with cHL (as confirmed via expert review) treated in the same studies served as reference cohorts. However, because individuals with stage IA NLPHL without clinical risk factors could not be included in the HD16 study, patients with cHL treated within the HD16 study for stage IA disease without clinical risk factors were excluded from the analysis to allow for a better comparability between the groups. Chemotherapy consisted of 2 cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD; HD16 study) or 2 cycles of escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) plus 2 cycles of ABVD (HD17 study). An iPET was performed after chemotherapy (patients who did not have an iPET performed per protocol, were not included in the analysis), the results of which were considered negative in case of a Deauville score (DS) of <3. In the experimental study arms, patients with a negative iPET result did not undergo consolidation RT, whereas patients with a negative iPET result assigned to the standard arms and patients with a positive iPET result received consolidation RT. Inclusion criteria and drug doses as well as RT fields and doses have been described elsewhere.16,17 The studies were approved by the review boards of the participating sites and were conducted in accordance with the Declaration of Helsinki.
Statistical methods
Progression-free survival (PFS) was defined as the time from completion of initial staging until disease progression, relapse, or death from any cause. If none of these events had occurred, PFS was censored at the date of last updates on the disease status. Overall survival (OS) was defined as the time from completion of initial staging until death from any cause and was censored at the date of last information updates for surviving patients. Time-to-event end points were analyzed using the Kaplan-Meier method and Cox regression analyses, including hazard ratios and 95% confidence intervals (CIs). Comparisons of patient characteristics and outcomes between patients with NLPHL and those with cHL were conducted using Fisher exact test and log-rank test, as applicable. Additional analyses including treatment group comparisons among patients with NLPHL were performed descriptively. SAS version 9.4 for Microsoft Windows (SAS Institute, Cary, NC) was used for all analyses.
Results
Patient characteristics
Overall, 85 patients with NLPHL who had treatment in the HD16 study for early-stage favorable HL were included in the present analysis (Figure 1A). They were compared with 495 patients with cHL treated in the same study. The median age was 37 years (range, 19-71 years) for patients with NLPHL and 36 years (range, 18-75 years) for patients with cHL (P = .438). Patients with NLPHL were male in 62 of 85 cases (72.9%), whereas males accounted for 254 of 495 patients with cHL (51.3%; P = .0002). Infradiaphragmatic disease was documented for 22 of 85 patients with NLPHL (25.9%) and 45 of 495 patients with cHL (9.1%; P < .0001). Information on the histopathological growth pattern was available for 67 of 85 patients with NLPHL (78.9%). Of these, the majority (44 of 67 patients [65.7%]) had a typical histopathological growth pattern (defined as growth pattern A or B according to Fan et al15).
CONSORT charts. (A) CONSORT chart for patients with NLPHL treated in the HD16 study. (B) CONSORT chart for patients with NLPHL treated in the HD17 study.
CONSORT charts. (A) CONSORT chart for patients with NLPHL treated in the HD16 study. (B) CONSORT chart for patients with NLPHL treated in the HD17 study.
A total of 15 patients with NLPHL treated in the HD17 study for early-stage unfavorable HL were included in this analysis and compared with 764 patients with cHL (Figure 1B). Patients with NLPHL had a median age of 42 years (range, 23-59 years), the median age of patients with cHL was 31 years (range, 18-60 years; P = .3967). Of the patients with NLPHL and those with cHL, 13 of 15 (86.7%) and 337 of 795 (44.1%), respectively, were male (P = .0011). Involvement of ≥3 nodal areas was the most common GHSG risk factor for both patients with NLPHL (14 of 15 patients [93.3%]) and cHL (556 of 764 patients [72.8%]; P = .0834). Patients with NLPHL presented with infradiaphragmatic disease in 7 of 15 cases (46.7%) as compared with 43 of 764 of patients with cHL (5.6%; P < .0001). Information on the histopathological growth pattern was available for 10 of 15 patients with NLPHL (66.7%). The majority had a typical histopathological growth pattern (7 of 10 patients [70%]; Table 1).
Characteristics of patients with NLPHL and patients with cHL treated in the HD16 and HD17 studies
| . | HD16 . | HD17 . | ||||
|---|---|---|---|---|---|---|
| NLPHL (n = 85) . | cHL (n = 495) . | P value . | NLPHL (n = 15) . | cHL (n = 764) . | P value . | |
| Median age, y (range) | 37 (19-71) | 36 (18-75) | .4380 | 42 (23-59) | 31 (18-60) | .3967 |
| Males | 62 (72.9%) | 254 (51.3%) | .0002 | 13 (86.7%) | 337 (44.1%) | .0011 |
| Stage II | 81 (95.3%) | 464 (93.7%) | .8051 | 15 (100%) | 720 (94.2%) | 1.0000 |
| B symptoms | 12 (14.1%) | 64 (12.9%) | .7298 | 2 (13.3%) | 189 (24.7%) | .5433 |
| ECOG PS 0 | 81 (95.3%) | 452 (91.3%) | .2829 | 15 (100%) | 635 (83.1%) | .1502 |
| Infradiaphragmatic disease | 22 (25.9%) | 45 (9.1%) | <.0001 | 7 (46.7%) | 43 (5.6%) | <.0001 |
| Mediastinal involvement | 5 (5.9%) | 183 (37%) | <.0001 | 4 (26.7%) | 674 (88.2%) | <.0001 |
| Large mediastinal mass | — | — | — | 0 (0%) | 137 (17.9%) | .0868 |
| Extranodal involvement | — | — | — | 2 (13.3%) | 57 (7.5%) | .3162 |
| Involvement of ≥3 nodal areas | — | — | — | 14 (93.3%) | 556 (72.8%) | .0834 |
| Elevated ESR | — | — | — | 0 (0%) | 350 (45.8%) | .002 |
| Typical growth pattern | 44 of 67 (65.7%) | — | — | 7 of 10 (70%) | — | — |
| Positive interim PET | 39 (45.9%) | 171 (34.5%) | .0507 | 3 (20%) | 246 (32.2%) | .4097 |
| DS 3 | 18 of 39 (46.2%) | 109 of 171 (63.7%) | 2 of 3 (66.7%) | 181 of 246 (73.6%) | ||
| DS 4 | 21 of 39 (53.8%) | 62 of 171 (36.3%) | 1 of 3 (33.3%) | 65 of 246 (26.4%) | ||
| . | HD16 . | HD17 . | ||||
|---|---|---|---|---|---|---|
| NLPHL (n = 85) . | cHL (n = 495) . | P value . | NLPHL (n = 15) . | cHL (n = 764) . | P value . | |
| Median age, y (range) | 37 (19-71) | 36 (18-75) | .4380 | 42 (23-59) | 31 (18-60) | .3967 |
| Males | 62 (72.9%) | 254 (51.3%) | .0002 | 13 (86.7%) | 337 (44.1%) | .0011 |
| Stage II | 81 (95.3%) | 464 (93.7%) | .8051 | 15 (100%) | 720 (94.2%) | 1.0000 |
| B symptoms | 12 (14.1%) | 64 (12.9%) | .7298 | 2 (13.3%) | 189 (24.7%) | .5433 |
| ECOG PS 0 | 81 (95.3%) | 452 (91.3%) | .2829 | 15 (100%) | 635 (83.1%) | .1502 |
| Infradiaphragmatic disease | 22 (25.9%) | 45 (9.1%) | <.0001 | 7 (46.7%) | 43 (5.6%) | <.0001 |
| Mediastinal involvement | 5 (5.9%) | 183 (37%) | <.0001 | 4 (26.7%) | 674 (88.2%) | <.0001 |
| Large mediastinal mass | — | — | — | 0 (0%) | 137 (17.9%) | .0868 |
| Extranodal involvement | — | — | — | 2 (13.3%) | 57 (7.5%) | .3162 |
| Involvement of ≥3 nodal areas | — | — | — | 14 (93.3%) | 556 (72.8%) | .0834 |
| Elevated ESR | — | — | — | 0 (0%) | 350 (45.8%) | .002 |
| Typical growth pattern | 44 of 67 (65.7%) | — | — | 7 of 10 (70%) | — | — |
| Positive interim PET | 39 (45.9%) | 171 (34.5%) | .0507 | 3 (20%) | 246 (32.2%) | .4097 |
| DS 3 | 18 of 39 (46.2%) | 109 of 171 (63.7%) | 2 of 3 (66.7%) | 181 of 246 (73.6%) | ||
| DS 4 | 21 of 39 (53.8%) | 62 of 171 (36.3%) | 1 of 3 (33.3%) | 65 of 246 (26.4%) | ||
ECOG PS, Eastern Cooperative Oncology Group performance status; ESR, erythrocyte sedimentation rate.
PET-2 results and PFS
In the HD16 study, 39 of 85 patients with NLPHL (45.9%) had a positive iPET as compared with 171 of 495 patients with cHL (34.5%; P = .0507). In the HD17 study, the iPET result was positive for 3 of 15 patients with NLPHL (20%) and for 246 of 764 patients with cHL (32.2%; P = .4097; Table 1).
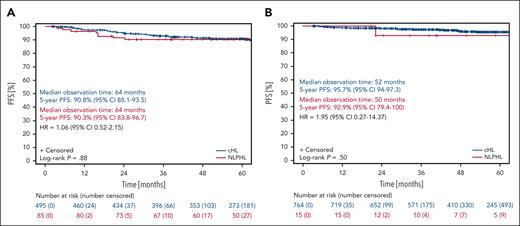
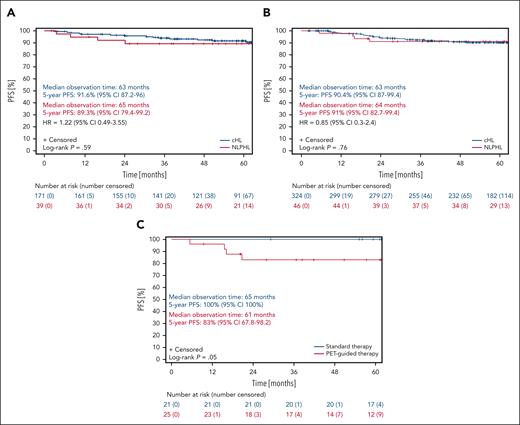
With regard to PFS, patients with NLPHL and patients with cHL treated within the HD16 study had a median observation time of 64 months. The 5-year PFS rates for patients with NLPHL and cHL were 90.3% (95% CI, 83.8-96.7) and 90.8% (95% CI, 88.1-93.5), respectively (P = .88; Figure 2A). Patients with NLPHL with a positive iPET result had a 5-year PFS of 89.3% (95% CI, 79.4-99.2), whereas patients with cHL with a positive iPET result had a 5-year PFS of 91.6% (95% CI, 87.2-96; P = .59; Figures 3A and 4A). The 5-year PFS for patients with NLPHL with a negative iPET result was 91% (95% CI, 82.7-99.4), and their counterparts with cHL had a 5-year PFS of 90.4% (95% CI, 87-99.4; P = .76; Figures 3B and 4A). Patients with NLPHL with a negative iPET result receiving chemotherapy plus consolidation RT (n = 21) had a 5-year PFS of 100% (95% CI, 100) as compared with 83% (95% CI, 67.8-98.2) for patients with NLPHL with a negative iPET result who did not undergo consolidation RT (n = 25; P = .05; Figure 3C). If iPET positivity was not defined as a DS >2 but as a DS >3, the 5-year PFS rates would be 85% (95% CI, 69.4-100) for patients with NLPHL with a positive iPET result and 91.9% (95% CI, 85.1-98.7) for patients with NLPHL with a negative iPET result (P = .46; Figure 4B).
Progression-free survival of NLPHL and cHL patients treated within the HD16 and HD17 studies. (A) PFS of patients with NLPHL (red) and those with cHL (blue) treated in the HD16 study. (B) PFS of patients with NLPHL (red) and those with cHL (blue) treated in the HD17 study.
Progression-free survival of NLPHL and cHL patients treated within the HD16 and HD17 studies. (A) PFS of patients with NLPHL (red) and those with cHL (blue) treated in the HD16 study. (B) PFS of patients with NLPHL (red) and those with cHL (blue) treated in the HD17 study.
Progression-free survival of NLPHL and cHL patients from the HD16 study according to the iPET result. (A) PFS of patients with NLPHL (red) and those with cHL (blue) from the HD16 study with a positive iPET result. (B) PFS of patients with NLPHL (red) and those with cHL (blue) from the HD16 study with a negative iPET result. (C) PFS of patients with NLPHL from the HD16 study with a negative iPET result who had chemotherapy alone (red) or combined-modality treatment (blue).
Progression-free survival of NLPHL and cHL patients from the HD16 study according to the iPET result. (A) PFS of patients with NLPHL (red) and those with cHL (blue) from the HD16 study with a positive iPET result. (B) PFS of patients with NLPHL (red) and those with cHL (blue) from the HD16 study with a negative iPET result. (C) PFS of patients with NLPHL from the HD16 study with a negative iPET result who had chemotherapy alone (red) or combined-modality treatment (blue).
Progression-free survival of NLPHL patients from the HD16 study according to the cut-off for iPET positivity. PFS of patients with NLPHL from the HD16 study with either a positive or negative iPET result: if a DS <3 is considered as an iPET-negative result (A) and if a DS <4 is considered as an iPET-negative result (B). iPET, PET-2.
Progression-free survival of NLPHL patients from the HD16 study according to the cut-off for iPET positivity. PFS of patients with NLPHL from the HD16 study with either a positive or negative iPET result: if a DS <3 is considered as an iPET-negative result (A) and if a DS <4 is considered as an iPET-negative result (B). iPET, PET-2.
In the HD17 study, the median observation time with regard to PFS was 50 months for patients with NLPHL and 52 months for patients with cHL. The 5-year PFS rates for patients with NLPHL and cHL were 92.9% (95% CI, 79.4-100) and 95.7% (95% CI, 94-97.3), respectively (P = .50; Figure 2B). For individuals with NLPHL treated within the HD17 study, PFS rates according to the iPET results were not investigated because of the small number of patients.
NLPHL recurrence, second primary malignancies, and OS
A total of 9 patients with NLPHL treated in the HD16 study had disease recurrence during follow-up. Of these patients, 3 had initially presented with a typical histopathological growth pattern, 4 had a variant histology, and 2 had an unknown histopathological growth pattern. The median time to relapse, defined as the time between the end of the first-line treatment and disease recurrence, was 14 months (range, 1-89 months). Second-line treatment consisted of high-dose chemotherapy and autologous stem cell transplantation (ASCT) (n = 3); RT alone (n = 2); single-agent ibrutinib (n = 2); and rituximab, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (R-BEACOPP) (n = 1), respectively (for 1 patient, second-line treatment was not documented). Both patients who had single-agent ibrutinib as salvage therapy developed a second relapse and received third-line treatment with single-agent rituximab and high-dose chemotherapy followed by ASCT (Table 2). Second primary malignancies occurred in 2 patients with NLPHL treated in the HD16 study. One patient was diagnosed with malignant melanoma, and 1 patient with stomach cancer. None of these patients had NLPHL recurrence before the occurrence of the second primary malignancy. There was no case of histological transformation into aggressive B-NHL (data not shown).
Relapse characteristics of patients with NLPHL treated in the HD16 and HD17 studies
| Study . | Gender . | Growth pattern at initial diagnosis . | NLPHL localization at initial diagnosis . | DS at iPET . | RT at first-line treatment . | Localization of relapse . | Second-line treatment . | Third-line treatment . |
|---|---|---|---|---|---|---|---|---|
| HD16 | Male | Typical | Supradiaphragmatic | 2 | No | IIS | RT alone | — |
| HD16 | Female | Typical | Supradiaphragmatic | 1 | No | IIS | Ibrutinib | Rituximab alone |
| HD16 | Female | Typical | Infradiaphragmatic | 3 | Yes | DiS | n/a | — |
| HD16 | Male | Variant | Supradiaphragmatic | 1 | No | IIS and DiS | HDCT + ASCT | — |
| HD16 | Male | Variant | Supradiaphragmatic | 4 | Yes | IIS and DiS | HDCT + ASCT | — |
| HD16 | Female | Variant | Infradiaphragmatic | 2 | No | IIS | RT alone | — |
| HD16 | Male | Variant | Infradiaphragmatic | 4 | Yes | DiS | R-BEACOPP | — |
| HD16 | Male | n/a | Supradiaphragmatic | 3 | Yes | DiS | Ibrutinib | HDCT + ASCT |
| HD16 | Male | n/a | Supradiaphragmatic | 4 | No∗ | IIS | HDCT + ASCT | — |
| HD17 | Male | n/a | Infradiaphragmatic | 4 | Yes | IIS | HDCT + ASCT | — |
| Study . | Gender . | Growth pattern at initial diagnosis . | NLPHL localization at initial diagnosis . | DS at iPET . | RT at first-line treatment . | Localization of relapse . | Second-line treatment . | Third-line treatment . |
|---|---|---|---|---|---|---|---|---|
| HD16 | Male | Typical | Supradiaphragmatic | 2 | No | IIS | RT alone | — |
| HD16 | Female | Typical | Supradiaphragmatic | 1 | No | IIS | Ibrutinib | Rituximab alone |
| HD16 | Female | Typical | Infradiaphragmatic | 3 | Yes | DiS | n/a | — |
| HD16 | Male | Variant | Supradiaphragmatic | 1 | No | IIS and DiS | HDCT + ASCT | — |
| HD16 | Male | Variant | Supradiaphragmatic | 4 | Yes | IIS and DiS | HDCT + ASCT | — |
| HD16 | Female | Variant | Infradiaphragmatic | 2 | No | IIS | RT alone | — |
| HD16 | Male | Variant | Infradiaphragmatic | 4 | Yes | DiS | R-BEACOPP | — |
| HD16 | Male | n/a | Supradiaphragmatic | 3 | Yes | DiS | Ibrutinib | HDCT + ASCT |
| HD16 | Male | n/a | Supradiaphragmatic | 4 | No∗ | IIS | HDCT + ASCT | — |
| HD17 | Male | n/a | Infradiaphragmatic | 4 | Yes | IIS | HDCT + ASCT | — |
IIS, initially involved site(s); DiS, distant site(s); HDCT, high-dose chemotherapy; n/a, not available; R-BEACOPP, rituximab, bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone
This patient did not receive RT at the local investigator’s discretion despite a positive iPET result.
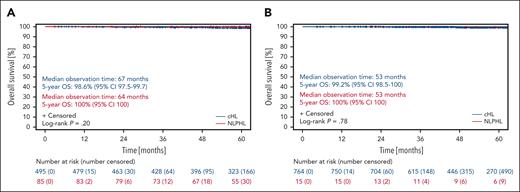
With regard to OS, patients with NLPHL and those with cHL treated in the HD16 study had median observation times of 64 and 67 months, respectively. The 5-year OS rates were 100% (95% CI, 100) for patients with NLPHL and 98.6% (95% CI, 97.5-99.7) for patients with cHL (P = .20; Figure 5A).
Overall survival of NLPHL and cHL patients treated within the HD16 and HD17 studies. (A) OS of patients with NLPHL (red) and patients with cHL (blue) treated in the HD16 study. (B) OS of patients with NLPHL (red) and patients with cHL (blue) treated in the HD17 study.
Overall survival of NLPHL and cHL patients treated within the HD16 and HD17 studies. (A) OS of patients with NLPHL (red) and patients with cHL (blue) treated in the HD16 study. (B) OS of patients with NLPHL (red) and patients with cHL (blue) treated in the HD17 study.
There was 1 case of disease recurrence among the patients with NLPHL treated in the HD17 study. The time to relapse was 16 months. Second-line treatment consisted of high-dose chemotherapy and ASCT (Table 2). There were no cases of second primary malignancies or histological transformation into aggressive B-NHL (data not shown). In the HD17 study, the median observation time with regard to OS was 53 months for patients with NLPHL and those with cHL. The 5-year OS rates were 100% (95% CI, 100) for patients with NLPHL and 99.2% (95% CI, 98.5-100) for patients with cHL (P = .78; Figure 5B).
Discussion
To our knowledge, this is one of the largest reports on patients with early-stage NLPHL. It is also, probably, the first analysis evaluating the role of iPET in individuals with early-stage NLPHL who had treatment in prospective randomized studies. The major findings evolving from the analysis are as follows: (1) characteristics of patients with early-stage NLPHL partly differ from those of their counterparts with cHL; (2) omission of consolidation RT after 2 cycles of ABVD in patients with early-stage favorable NLPHL with a negative iPET results in a strong trend toward an increased relapse rate; (3) with 5-year PFS rates >90% and a 5-year OS rate of 100%, contemporary HL-directed treatment approaches result in excellent outcomes for patients with early-stage NLPHL.
In this analysis, patients with NLPHL diagnosed in early favorable and early unfavorable stages had a median age of 37 and 42 years, respectively. Most patients with NLPHL were male (early favorable stages: 72.9%; early unfavorable stages: 86.7%), and the majority presented with a typical histopathological growth pattern (early favorable stages: 65.7%; early unfavorable stages: 70%). Hence, patient characteristics were consistent with those of previous reports on individuals with stage I/II NLPHL. An older analysis using the British Columbia Cancer Agency (BCCA) database included 88 patients with limited-stage NLPHL. The median age was 36.5 years, and 75% of the patients were male.18 A more recent multi-institutional retrospective analysis conducted by the International Lymphoma Radiation Oncology Group (ILROG) included 559 patients with stage I/II NLPHL with a median age of 39 years; the proportion of males was 72.3%. The histopathological growth pattern was reported for 203 patients. Of these, 166 (79.4%) had a typical histopathological growth pattern.11
In this analysis, the iPET at the end of chemotherapy was positive in 45.9% and 20% of patients with NLPHL diagnosed in early favorable and early unfavorable stages, respectively. A previous retrospective analysis using the BCCA database included 63 patients with limited-stage NLPHL who had undergone iPET after 2 cycles of ABVD; the iPET result had been positive in 14% of cases.14 The lower rate of patients with a positive iPET result in the earlier analysis may, at least in part, be because of different cut-off values for iPET positivity. A DS of 3 was considered as an iPET-negative result in the analysis using the BCCA database but as an iPET-positive result in the HD16 and HD17 studies. If a DS of 3 had been considered as an iPET-negative result in the HD16 and HD17 studies as well, only 21 of 85 patients with NLPHL (24.7%) from the HD16 study and 1 of 15 patients with NLPHL (6.7%) from the HD17 study would have had a positive iPET result. The exclusion of individuals with stage IA NLPHL without clinical risk factors from the HD16 study and, thus, also from this analysis might have also contributed to the different proportions of patients with a positive iPET result.
The 5-year PFS rates for patients with NLPHL treated in the HD16 and HD17 studies were 90.3% and 92.9%, respectively. This is in line with a previous multi-institutional analysis from the ILROG that reported a 5-year PFS rate of 87.1% for patients with stage I/II NLPHL. Taking into account this previous analysis, treatment approaches applied to patients were heterogeneous and included combined-modality treatment, chemotherapy alone, RT alone, and rituximab-containing therapies mirroring varying preferences at different institutions and the large time interval from 1995 to 2018 during which patients had been treated.11 The question of whether one approach should be preferred over the other in the treatment of patients with early-stage NLPHL has not been answered until now. The same is true for the question of whether these patients benefit from rituximab-containing treatment strategies. Prospective phase 2 studies evaluating single-agent rituximab in patients with newly diagnosed NLPHL indicated a response rate of 100%.19,20 However, >50% of patients relapsed within 5 years, so disease control with rituximab alone appears to be worse than that with approaches including conventional chemotherapy and/or RT.20 A small, single-center, retrospective analysis including a total of 28 patients with NLPHL who either received rituximab-containing therapy (consisting of rituximab alone for early-stage favorable disease, 4 cycles of ABVD plus rituximab for early-stage unfavorable disease, and 6 cycles of ABVD plus rituximab for advanced-stage disease) or rituximab-free approaches (4 cycles of ABVD plus RT for early-stage favorable disease and early-stage unfavorable disease, and 6 cycles of ABVD for advanced-stage disease) revealed an improved 7-year PFS with rituximab-containing treatment only for patients with advanced-stage disease.21 However, final conclusions cannot be drawn from this analysis because of the small number of patients. Studies allowing firm conclusions with regard to the role of B-NHL–directed protocols in patients with early-stage NLPHL are also pending.
In this analysis, patients with early-stage favorable NLPHL who had a negative iPET result after 2 cycles of ABVD and did not receive consolidation RT showed a strong trend toward an impaired PFS in comparison with patients who had a negative iPET result and had received consolidation RT. This is in agreement with the results for the whole HD16 study population. The final analysis of the study indicated a significant loss of tumor control for patients who had a negative iPET result for whom consolidation RT had been omitted.16 A retrospective study from the Lymphoma Study Association including 314 patients with NLPHL of all stages who were managed with different approaches also demonstrated an improved 4-year PFS after combined-modality treatment as compared with other treatment strategies. Patients included in this previous analysis were not treated on the basis of the result of an iPET.22
A total of 10 patients with NLPHL treated within the HD16 and HD17 studies experienced disease recurrence during follow-up. Second-line treatment varied considerably ranging from low-intensity approaches such as RT alone and single-agent ibrutinib (given within a phase 2 study for patients with relapsed NLPHL) to high-dose chemotherapy and ASCT.23 This diversity of therapies is consistent with earlier analyses on patients with relapsed NLPHL. Among 99 patients with NLPHL recurrence included in an analysis from the GHSG, only 31% received intensive salvage treatment with high-dose chemotherapy and ASCT whereas 37% had second-line treatment with either single-agent rituximab or RT alone.24 Similarly, in patients with relapsed NLPHL that were investigated in a retrospective study from North America, only 20% of cases had received high-dose chemotherapy and ASCT.25 The outcomes in both the GHSG analysis and the study from North America were excellent irrespective of the applied salvage therapy, with 5-year PFS-2 rates of 75.6% and 68%, respectively.24,25
In this analysis, patients with NLPHL had a 5-year OS of 100%. Survival data reported previously were comparable with these results. A large retrospective study from the ILROG indicated a 5-year OS rate of 98.3%.11 An analysis using the BCCA database included 99 patients with limited-stage NLPHL and revealed a 5-year OS rate of 97%.14 The 10-year OS rates for patients with early-stage favorable and early-stage unfavorable NLPHL who had treatment within the randomized GHSG HD7-HD15 studies were 93.3% and 96.2%, respectively.9
Limitations of this analysis include the follow-up duration. The median observation time of ∼5 years does not allow final conclusions with regard to disease control because patients with NLPHL tend to develop late relapses. A previous GHSG analysis of relapsed NLPHL revealed a median time to disease recurrence of 3.7 years.24 An even longer median time to NLPHL recurrence of 5.2 years was demonstrated for patients with a typical histopathological growth pattern at initial diagnosis.26 It was also not possible to investigate the impact of different cut-off values for iPET positivity. Within the HD16 and HD17 studies, a DS >2 was consistently considered as an iPET-positive result so that all patients with a DS of 3 or 4 had consolidation RT. In contrast, the current definition of iPET positivity in clinical practice is not a DS >2 but a DS >3.
In conclusion, this study indicates that contemporary HL-directed treatment approaches are highly active in newly diagnosed early-stage NLPHL. In patients with early-stage favorable NLPHL, the addition of RT to a brief chemotherapy with 2 cycles of ABVD appears necessary irrespective of the iPET result. Future studies are needed to define whether ABVD represents the optimal chemotherapy protocol for all patients with early-stage NLPHL and to clarify the role of anti-CD20 antibodies in this patient group.
Acknowledgment
S.H. is supported by the Deutsche Forschungsgemeinschaft HA6145/7-1.
Authorship
Contribution: D.A.E., I.B., S.H., A.E., and P.B. designed the research, and analyzed and interpreted the data; D.A.E. and I.B. wrote the paper; and all authors provided study material or recruited patients and gave the final approval of the manuscript.
Conflict-of-interest disclosure: D.A.E. reports honoraria from Sanofi-Genzyme and Takeda. B.v.T. reports grants or contracts from Novartis, Takeda, and MSD; reports consulting fees from Allogene, BMS/Celgene, Cerus, Incyte, IQVIA, Miltenyi, Novartis, Pentixapharm, Roche, Amgen, Pfizer, Takeda, MSD, and Kite/Gilead; reports honoraria from AstraZeneca, BMS, Incyte, Novartis, Roche, Takeda, and MSD; and reports support for attending meetings and/or travel from AbbVie, AstraZeneca, Kite/Gilead, MSD, Roche, Takeda, and Novartis. A.E. reports honoraria or research funding from AstraZeneca, Bristol Myers Squibb, Hexal AG, Innovent, Janssen-Cilag, MSD, Takeda, Tessa Pharma, and TS Oncology. The remaining authors declare no competing financial interests.
Correspondence: Dennis A. Eichenauer, First Department of Internal Medicine, University Hospital Cologne, Kerpener Str 62, D-50937 Cologne, Germany; e-mail: dennis.eichenauer@uk-koeln.de.
References
Author notes
Presented in abstract form at the 63rd Annual Meeting of the American Society of Hematology, Atlanta, GA, 11 to 14 December 2021, and the 12th International Symposium on Hodgkin Lymphoma, Cologne, Germany, 22 to 24 October 2022.
Research data will be available from the corresponding author, Dennis A. Eichenauer (dennis.eichenauer@uk-koeln.de), on request with a reasonable project outline.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal