In this issue of Blood, Dreval et al1 attempted to unravel the basis of follicular lymphoma (FL) transformation using whole genome sequencing, comparing cases with and without histologic transformation (HT and no HT), with de novo diffuse large B-cell lymphoma (DLBCL). FL shares many common genetic events with the germinal center subtype (GCB) of DLBCL; thus, the authors reasoned that DNA from de novo DLBCL would be enriched for mutations associated with HT, and an ideal group to mine for predictive genes. To sort these subtypes, the authors employed a multistep process defining significantly mutated genes and activation-induced cytidine deaminase (AID)–dependent mutational signatures within the cohort and then searched for differences between lymphoma subtypes. In doing so, they discovered 2 genetically distinct subgroups of FL, DLCBL-like FL (dFL) and constrained FL (cFL), that showed a remarkable 10-year difference in time to transformation.
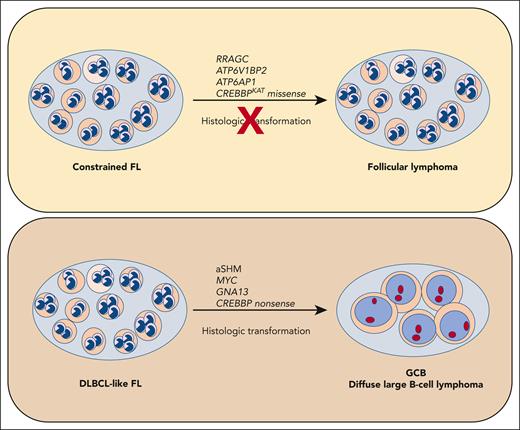
These 2 FL subtypes demonstrated differences in mutation signatures, mutated gene frequencies, gene expression, and translocations. For instance, the cFL samples showed a lower mutational burden for hotspots of aberrant somatic hypermutation (aSHM) induced by AID, greater enrichment of mutations in RRAGC, ATP6V1BP2, and ATP6AP1, and missense mutations in CREBBP (see figure). cFL cases had lower gene expression of MYC, and lower frequency of MYC translocations. Conversely, the dFL cases had a higher incidence of aSHM, as evidenced by mutations in the transcription start sites of BCL6, BCL7A, RHOH, and ZFPL36L1. Moreover, dFL shared a similar mutation pattern with DLCBL, including a higher incidence of GNA13 mutations and higher gene expression of MYC compared with cFL.
Whole genome sequencing identified 2 subtypes of FL with differing risks of histologic transformation. Aberrant somatic hypermutation (aSHM) induced by AID was associated with increased risk of transformation and increased number of mutations shared with GCB DLCBL. This subset was named DLBCL-like FL (dFL). Conversely, the cohort with lower risk of transformation showed less aSHM and fewer overall mutations, and enrichment for missense mutations of CREBBP, ATP6V1BP2, ATP6AP1, and RRAGC. This subset was named constrained FL (cFL).
Whole genome sequencing identified 2 subtypes of FL with differing risks of histologic transformation. Aberrant somatic hypermutation (aSHM) induced by AID was associated with increased risk of transformation and increased number of mutations shared with GCB DLCBL. This subset was named DLBCL-like FL (dFL). Conversely, the cohort with lower risk of transformation showed less aSHM and fewer overall mutations, and enrichment for missense mutations of CREBBP, ATP6V1BP2, ATP6AP1, and RRAGC. This subset was named constrained FL (cFL).
The overall similarity of mutation patterns between cFL, dFL, and GCB DLBCL underscores the genetic relationship between these diseases and further supports the notion that FL represents the indolent counterpart of the EZH2 mutant, BCL2 translocated genetic subtype of DLBCL.2 However, a notable finding was the enrichment of missense mutations in the lysine acetyltransferase (KAT) domain of CREBBP within the cFL cases vs nonsense and frameshift mutations in the dFL and DLBCL cases. The authors postulate that the missense mutations in CREBBP (and higher gene expression) in cFL are incompatible with HT, and possibly limit other mechanisms of transformation, including aSHM and higher MYC activity. Previous studies using mouse models of FL/DLCBL noted similar findings, suggesting that higher MYC expression and more aggressive disease were associated with complete inactivation of Crebbp.3 The authors were able to demonstrate that the presence or absence of CREBBP KAT domain missense mutations alone was able to maintain the significant association with time to transformation in 2 separate clinical cohorts.4,5 Thus, CREBBP missense mutations may reflect the hallmark genetic feature of truly indolent FL and could be studied as a prospective biomarker for clinical decisions for treatment vs watch and wait.
Other tools to predict poor prognosis FL have been developed, including the FL international prognostic index (FL-IPI),6 the m7-FLIPI7 (a clinical and genetic multivariate risk model), and progression of disease within 2 years (POD24).8 How does the current study of genetic subtypes compare? A direct comparison is difficult because the above risk models predict progression-free survival, whereas the new genetic subtypes predict time to transformation. Although no difference in FL-IPI scores or POD24 status was observed between cFL and dFL, Dreval et al demonstrate that cFL/dFL designation is an independent risk model and complements both the FL-IPI and POD24 in identifying high-risk patients. Interestingly, the dFL subtype showed a near 3-fold enrichment of grade 3A FL cases. This finding suggests value in the traditional histologic grading system based on centroblasts per high-power field and supports a potential difference in the mutation patterns among FL grades.
Other large-scale sequencing studies in FL have also recently been published.5 Crouch et al used a panel of nearly 300 genes and reported 3 genetic subtypes that differed significantly with regard to prevalence of aSHM, but the subtypes did not correlate to FLIPI or histologic transformation. However, differences in aSHM were observed between subgroups and CREBBP mutations clustered with STAT6 and TNFRSF14 mutations, both of which trended toward significance in the cFL subtype reported by Dreval et al. Crouch et al conclude that the genetic subtypes they found had limited prognostic clinical significance. How then do we reconcile these different conclusions? First, the approaches were different. Crouch et al used panel deep sequencing (∼500×) of formalin-fixed, paraffin-embedded FL biopsies, whereas Dreval et al used whole genome sequencing (∼50×) with paired normal from newly sequenced and historic cohorts of FL and DLBCL. Thus, the patient populations, controls, and genomic features detected for the 2 models were different. Second, of the overlapping genetic features, CREBBP mutations were all treated similarly by Crouch et al, whereas Dreval et al distinguished missense mutations within the KAT domain from other mutations. This small but important distinction appears to be a critical nuance in predicting transformation of FL. The distinction of domain-specific genetic features is also critical for predicting genetic subtypes of DLBCL.2 However, like the FL predictor, the full complement of genetic features is required to achieve maximum sensitivity, specificity, and accuracy. Future studies with large replication cohorts will be needed to establish the minimum set of genetic alterations required to appropriately predict genetic subtypes of lymphoma.
As machine learning approaches continue to gain traction and offer new insights into the heterogeneity of FL, it will be critical to thoroughly annotate the research cohorts being studied. Roughly 20% of transformed FL cases are of the activated B-cell (ABC) type by gene expression and have a lower incidence of BCL2 rearrangement, compared with GCB cases.4 Prior studies also have identified subsets of FL that more closely resemble ABC-DLBCL, with high expression of interferon regulatory factor 4 (IRF4) and low expression of CD10; high expression of IRF4 is a risk for early transformation.4,9 Notably, such cases are generally negative for the BCL2 translocation and should be distinguished from conventional FL. Recognizing that FL is >1 disease, we must ensure that future models take this heterogeneity into consideration.10
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal