In this issue of Blood, Shao et al1 reveal that ZDHHC21-mediated palmitoylation regulates oxidative phosphorylation and is essential for a subset of acute myeloid leukemia (AML) cells.
Oxidative phosphorylation is a mitochondrial metabolic process by which cells generate energy in the form of adenosine triphosphate (ATP). Electrons generated through the flux of metabolites through the tricarboxylic acid (TCA) cycle are transferred through a cascade of proteins termed respiratory chain complexes. The flow of electrons generates a negative electrical gradient in the inner mitochondrial membrane, which powers the production of ATP from adenosine 5′-diphosphate and inorganic phosphate.
A subset of AML cells have unique mitochondrial characteristics resulting in an increased reliance on oxidative phosphorylation. These cells have increased mitochondrial mass, increased flux of metabolites into the TCA cycle,2 decreased spare reserve capacity,3 and metabolic inflexibility such that AML cells cannot upregulate alternate energy production pathways to cope with reductions in oxidative phosphorylation.4
Given their heightened reliance on oxidative phosphorylation, strategies that target this process directly through respiratory chain complex inhibitors or indirectly by targeting mitochondrial DNA replication, mitochondrial protein synthesis, or mitochondrial proteases can selectively eradicate leukemic cells and leukemic stem cells preferentially over normal hematopoietic cells in vitro and in vivo.5,6 Although targeting oxidative phosphorylation directly by inhibiting respiratory chain complexes may lack an adequate therapeutic window,7 other strategies that capitalize on other mitochondrial and metabolic vulnerabilities in the leukemic cells and indirectly target oxidative phosphorylation seem more promising.8
Despite the importance of mitochondrial pathways and metabolism for a subset of AML cells, the molecular drivers of this phenotype are largely unknown. For example, increased reliance on oxidative phosphorylation does not correlate with specific cytogenetic or molecular mutations associated with AML. So, other causes must contribute, and it is important to understand these molecular regulators.
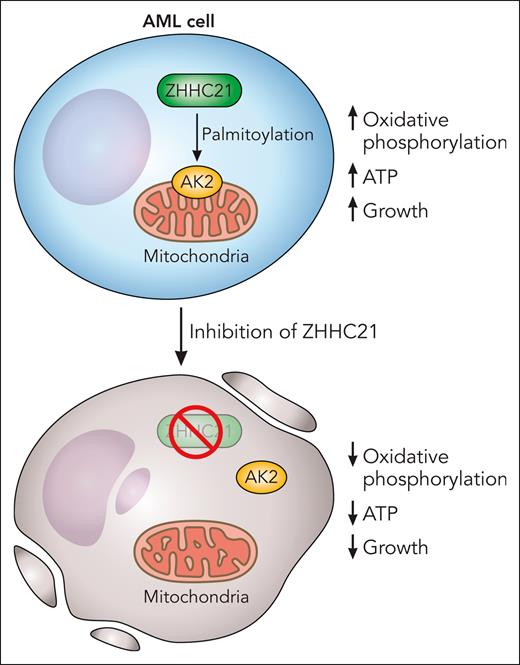
In an elegant and important study, Shao and colleagues searched for posttranslational protein modifications that impact oxidative phosphorylation in AML cells preferentially over normal hematopoietic cells. Through their screen, they discovered that S-palmitoylation by the palmitoyltransferase ZDHHC21 regulated oxidative phosphorylation in AML. Palmitoylation is a posttranslational modification whereby fatty acids such as palmitic acid are added to cysteine residues on proteins. The authors also mapped the protein substrates that were palmitoylated and interacted with ZDHHC21 and identified the mitochondrial kinase adenylate kinase (AK2) as a top hit. The team discovered that palmitoylation of AK2 promotes the mitochondrial localization of the kinase and controls oxidative phosphorylation and ATP production. Inhibiting ZDHHC21 with genetic and/or chemical approaches inhibited oxidative phosphorylation, reduced levels of ATP, and decreased the growth and viability of AML cells and stem cells in vitro and in vivo preferentially over normal hematopoietic cells (see figure).
Palmitoylation of target proteins, by the palmitoyltransferase ZHHC21, maintains oxidative phosphorylation and is necessary for the viability of AML cells and stem cells. AK2 is palmitoylated by ZHHC21, and AK2 palmitoylation is necessary for its localization to the mitochondria where it regulates oxidative phosphorylation. Chemical or genetic inhibition of ZHHC21 prevents the localization of AK2 to the mitochondria, decreases oxidative phosphorylation, reduces ATP, and inhibits the growth and viability of AML cells. Professional illustration by Patrick Lane, ScEYEnce Studios.
Palmitoylation of target proteins, by the palmitoyltransferase ZHHC21, maintains oxidative phosphorylation and is necessary for the viability of AML cells and stem cells. AK2 is palmitoylated by ZHHC21, and AK2 palmitoylation is necessary for its localization to the mitochondria where it regulates oxidative phosphorylation. Chemical or genetic inhibition of ZHHC21 prevents the localization of AK2 to the mitochondria, decreases oxidative phosphorylation, reduces ATP, and inhibits the growth and viability of AML cells. Professional illustration by Patrick Lane, ScEYEnce Studios.
This work by Shao et al is an important advance for several reasons. They uncovered new biology related to AML cells and stem cells and identified a molecular driver that regulates reliance on oxidative phosphorylation in this disease. In addition, they elegantly mapped how palmitoylation of protein substrates by ZDHHC21 influences mitochondrial function and specifically identified AK2 as a target. Finally, they discovered that ZDHHC21 controls oxidative phosphorylation, at least in part, by regulating the mitochondrial localization of AK2 through palmitoylation. Their work also has important therapeutic implications. They highlighted ZDHHC21 as a druggable potential therapeutic target in AML and the possibility of developing novel ZDHHC21 inhibitors in the future.
This work also raises new questions that would be important areas for future investigation. For example, which patients with AML are most sensitive to ZHHC21 inhibition and why? Some data by Shao et al suggest that patients with FMS-like tyrosine kinase 3-internal tandem duplication are particularly sensitive to ZHHC21 inhibition. Should future studies confirm these findings in larger cohorts, exploring why this subgroup is particularly sensitive will be important. In addition, is the increased reliance on ZDHHC21 specifically, and oxidative phosphorylation in general, a consequence or a driver of AML? Can potent and selective inhibitors of ZDHHC21 be developed? If so, what is their efficacy in AML and toxicity for normal cells? Thanks to Shao et al, we are now in a position to begin to ask and answer these questions.
Conflict-of-interest disclosure: A.D.S. has received research funding from Takeda Pharmaceuticals, Bristol Myers Squibb, and Medivir and consulting fees/honoraria from Bristol Myers Squibb, AstraZeneca, Takeda, Novartis, Jazz, and Otsuka Pharmaceuticals; and is named on a patent application for the use of double negative T cells to treat AML.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal