Key Points
Ineligibility rates were higher among Black patients and racial subgroups classified as Other.
Black patients were more likely to be ineligible for a trial due to failure to meet hematology laboratory criteria.
Abstract
The narrow eligibility criteria may contribute to the underrepresentation of racial and ethnic subgroups in cancer clinical trials. We conducted a retrospective pooled analysis of multicenter global clinical trials submitted to the US Food and Drug Administration between 2006 and 2019 to support the approval of the use of multiple myeloma (MM) therapies that analyze the rates and reasons for trial ineligibility based on race and ethnicity in MM clinical trials. Race and ethnicity were coded per Office of Management and Budget standards. Patients flagged as having screen failures were identified as ineligible. Ineligibility rates were calculated as the percentage of patients who were ineligible compared with the screened population within the respective racial and ethnic subgroups. Trial eligibility criteria were grouped into specific categories to analyze the reasons for trial ineligibility. Black patients (24%) and other (23%) race subgroups had higher ineligibility rates than White patients (17%). The Asian race had the lowest ineligibility rate (12%) among all racial subgroups. Failure to meet the hematologic laboratory criteria (19%) and treatment-related criteria (17%) were the most common reasons for ineligibility among Black patients and were more common in Black patients than in other races. Failure to meet disease-related criteria was the most common reason for ineligibility among White (28%) and Asian (29%) participants. Our analysis indicates that specific eligibility criteria may contribute to enrollment disparities for racial and ethnic subgroups in MM clinical trials. However, the small number of screened patients in the underrepresented racial and ethnic subgroups limits definitive conclusions.
Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy, with an estimated 34 470 new cases diagnosed in the United States in 2022.1 This disease affects African American people disproportionately. In the United States, African American people have a 2-times higher incidence of the disease than White people.2 Despite the higher incidence of the disease in African American people, this racial subgroup comprises <5% of the patients enrolled in MM registrational clinical trials.3 Similarly, there is a disparity between the incidence of MM and enrollment in clinical trials for the Hispanic population compared with the non-Hispanic White population.4 Differences exist in the biology and treatment patterns among racial and ethnic subgroups.5-8 Enrolling a diverse patient population in clinical trials supporting MM drug approval allows for an adequate understanding of the product’s therapeutic profile and the generalizability of the clinical trial data to patients who are most affected by the disease. A diverse study population may also allow for an understanding of the impact of biological differences that may result in differential responses to medical products across racial and ethnic populations. The lack of adequate representation of diverse racial and ethnic populations in clinical trials limits the evidence available to guide treatment and may affect the outcomes in racial and ethnic subgroups. Although studies have reported similar or improved survival in Black/African American patients compared with White patients with MM, particularly when access to care is similar,9 population studies indicate that African American patients have the highest mortality rates among all racial populations, followed by American Indian/Alaskan Native patients.10
There are many barriers to the enrollment of racial and ethnic minorities in clinical trials, including language barriers, socioeconomic status (including the lack of insurance), and difficulty with transportation.11,12 Restrictive eligibility criteria are an important barrier to the enrollment of patients in cancer clinical trials.13-15 Few studies have also reported specific eligibility criteria that may serve as a barrier to enrollment of underrepresented racial and ethnic populations.16-18 Although lower enrollment rates of racial and ethnic minority groups in MM clinical trials are well documented,3,4 there are limited data regarding eligibility criteria as a barrier to enrollment of racial and ethnic minorities in MM clinical trials.
In this article, we present our analysis of the rates and reasons for ineligibility based on race and ethnicity in MM clinical trials submitted to the US Food and Drug Administration (FDA) in support of marketing approval.
Methods
We reviewed FDA internal databases for clinical trials submitted to support approval for an MM indication between 2006 and 2019. Trials that did not collect any race/ethnicity data or reasons for screening failure were excluded. The baseline demographic characteristics of the clinical trial participants (age, sex, race, ethnicity, and country), eligibility status (eligible vs ineligible), and reasons for trial ineligibility were collected and standardized in the pooled data set. Data aggregation and analyses were conducted in R (R Core Team, 2019). The data extracted from individual trials were mapped onto a combined data set.
Race and ethnicity data were abstracted from clinical trial data sets and case report forms (CRFs) were submitted along with the clinical trial data. Race and ethnicity were coded per the Office of Management and Budget standards19 to collect and present data on race and ethnicity. If the “other” category was selected in the CRF for race, this was reported as “other.” Because of the very small numbers within the individual categories, American Indian or Alaska Native subgroups were grouped with the “other” category to report all subsequent analyses. If race or ethnicity was collected in the trial but was missing for an individual participant, it was coded as unknown.
Patients who were flagged as having screen failures in the data sets were identified as ineligible. Patients who were ineligible and not enrolled were categorized as excluded, and those enrolled despite not meeting eligibility criteria were grouped as having protocol violations.
Ineligibility reasons were grouped into specific categories as described later in this article. Failure to meet the eligibility criteria for protocol-defined organ-specific laboratory values for renal function and hepatic function were categorized under renal function and hepatic function, respectively. Patients not meeting eligibility based on protocol-specified hemoglobin, platelet count, or absolute neutrophil count criteria were categorized under hematology laboratory criteria. The cardiac function category included patients who failed to meet the protocol-defined clinical cardiac or ejection fraction criteria. Patients not meeting eligibility due to a requirement for specific values of pulmonary function tests or a history of asthma or chronic obstructive pulmonary disease were categorized under pulmonary function. Patients not meeting the eligibility criteria because of lack of measurable disease or the presence of other disease conditions, such as plasma cell leukemia or amyloidosis, were categorized as ineligible due to the disease-related criteria. Reasons for not meeting treatment-related criteria included the lack of receipt of specified lines of therapy, eg, at least 3 prior lines of therapy, or patients not refractory to protocol-specified treatments required for enrollment. Patients who did not meet the respective protocol-specified eligibility criteria requirements for active infection, prior malignancy, HIV or hepatitis virus, or contraception requirements were categorized under the respective individual categories. All other eligibility criteria, including the required Eastern Cooperative Oncology Group performance status, ability to provide informed consent, etc, were categorized under other inclusion/exclusion criteria. Patients who withdrew after screening were categorized under the group called patient decision. If more than 1 reason for ineligibility was listed for an individual patient, it was counted separately within the individual ineligibility categories.
The demographic characteristics of all screened patients and their eligibility were summarized in the pooled population. We calculated the ineligibility rate as the percentage of patients who were ineligible compared with the screened population within the respective racial and ethnic subgroups. Similar rates were calculated for patients excluded from the trials. The reasons for ineligibility were summarized by race and ethnicity. Only descriptive statistics were calculated because of the small number of patients in the individual categories of race and ethnicity. A 5% difference was considered relevant for identifying differences in the eligibility criteria between the White racial group and other racial and ethnic groups.
Results
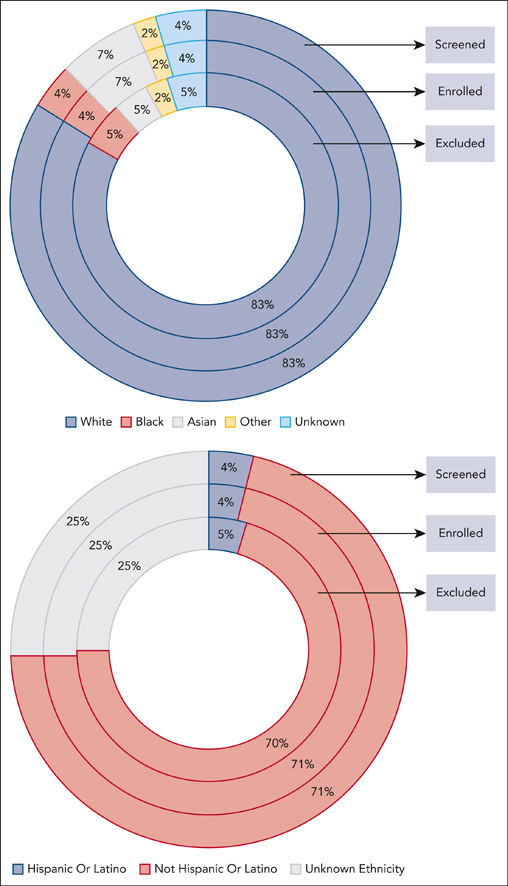
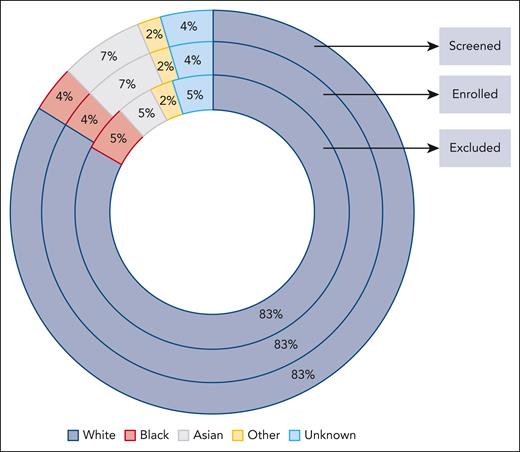
Sixteen trials (4 single-arm trials and 12 randomized clinical trials) with information on race and reasons for screen failures and ineligibility were pooled, providing a total of 9325 patients for inclusion in this analysis (Table 1). Of these, only 12% of the patients were screened in US sites compared with 88% in the rest of the world. Eighty-three percent of the patients screened were White, 7% were Asian, 4% were Black, 4% were of unknown race, and 2% were in the “other” category (American Indian or Alaska Native, Native Hawaiian, or other Pacific Islander) (Figure 1). Four percent of the patients screened reported Hispanic ethnicity, 71% reported non-Hispanic, and ethnicity was unknown for 25% (Figure 2).
Demographic characteristics of screened patients in the pooled data set
| . | Ineligible N = 1614 . | Eligible N = 7711 n (%) . | Total screened N = 9325 n (%) . | |
|---|---|---|---|---|
| Excluded N = 1342 n (%) . | Protocol violations N = 272 n (%) . | |||
| Age, median (range, y) | 69 [26, 92] | 67 [40, 91] | 67 [28, 93] | 67 [26, 93] |
| Age category | ||||
| <65 y | 416 (36) | 112 (41) | 3133 (41) | 3661 (40) |
| ≥65 to <75 | 445 (39) | 110 (41) | 3186 (41) | 3741 (41) |
| ≥75 | 294 (25) | 49 (18) | 1389 (18) | 1732 (19) |
| Sex | ||||
| Female | 615 (46) | 140 (51) | 3460 (45) | 4215 (45) |
| Male | 727 (54) | 132 (49) | 4251 (55) | 5110 (55) |
| Race | ||||
| White | 1118 (83) | 220 (81) | 6449 (84) | 7787 (84) |
| Black | 60 (4) | 28 (10) | 274 (4) | 362 (4) |
| Asian | 71 (5) | 2 (1) | 556 (7) | 629 (7) |
| Native Hawaiian or other Pacific Islander | 0 (0) | 0 (0) | 10 (<1) | 10 (<1) |
| American Indian or Alaskan Native | 2 (0) | 0 (0) | 4 (<1) | 6 (<1) |
| Unknown | 64 (5) | 11 (4) | 298 (4) | 373 (4) |
| Other | 27 (2) | 11 (4) | 120 (2) | 158 (2) |
| Ethnicity | ||||
| Hispanic or Latino | 63 (5) | 7 (3) | 278 (4) | 348 (4) |
| Not Hispanic or Latino | 946 (70) | 142 (52) | 5531 (72) | 6619 (71) |
| Unknown | 333 (25) | 123 (45) | 1902 (25) | 2358 (25) |
| Region | 301 (22) | 24 (9) | 795 (10) | 1120 (12) |
| US | 301 (22) | 24 (9) | 795 (10) | 1120 (12) |
| RoW | 1041 (78) | 248 (91) | 6916 (90) | 8205 (88) |
| Trial | ||||
| 1 | 0 (0) | 11 (4) | 918 (12) | 929 (10) |
| 2 | 0 (0) | 18 (7) | 460 (6) | 478 (5) |
| 3 | 45 (3) | 11 (4) | 123 (2) | 179 (2) |
| 4 | 33 (2) | 6 (2) | 103 (1) | 142 (2) |
| 5 | 125 (9) | 16 (6) | 553 (7) | 694 (7) |
| 6 | 99 (7) | 30 (11) | 468 (6) | 597 (6) |
| 7 | 181 (13) | 11 (4) | 696 (9) | 888 (10) |
| 8 | 215 (16) | 15 (6) | 722 (9) | 952 (10) |
| 9 | 0 (0) | 14 (5) | 708 (9) | 722 (8) |
| 10 | 115 (9) | 24 (9) | 622 (8) | 761 (8) |
| 11 | 40 (3) | 0 (0) | 117 (2) | 157 (2) |
| 12 | 139 (10) | 4 (1) | 451 (6) | 594 (6) |
| 13 | 140 (10) | 0 (0) | 646 (8) | 786 (8) |
| 14 | 46 (3) | 12 (4) | 114 (1) | 172 (2) |
| 15 | 0 (0) | 86 (32) | 232 (3) | 318 (3) |
| 16 | 164 (12) | 14 (5) | 778 (10) | 956 (10) |
| . | Ineligible N = 1614 . | Eligible N = 7711 n (%) . | Total screened N = 9325 n (%) . | |
|---|---|---|---|---|
| Excluded N = 1342 n (%) . | Protocol violations N = 272 n (%) . | |||
| Age, median (range, y) | 69 [26, 92] | 67 [40, 91] | 67 [28, 93] | 67 [26, 93] |
| Age category | ||||
| <65 y | 416 (36) | 112 (41) | 3133 (41) | 3661 (40) |
| ≥65 to <75 | 445 (39) | 110 (41) | 3186 (41) | 3741 (41) |
| ≥75 | 294 (25) | 49 (18) | 1389 (18) | 1732 (19) |
| Sex | ||||
| Female | 615 (46) | 140 (51) | 3460 (45) | 4215 (45) |
| Male | 727 (54) | 132 (49) | 4251 (55) | 5110 (55) |
| Race | ||||
| White | 1118 (83) | 220 (81) | 6449 (84) | 7787 (84) |
| Black | 60 (4) | 28 (10) | 274 (4) | 362 (4) |
| Asian | 71 (5) | 2 (1) | 556 (7) | 629 (7) |
| Native Hawaiian or other Pacific Islander | 0 (0) | 0 (0) | 10 (<1) | 10 (<1) |
| American Indian or Alaskan Native | 2 (0) | 0 (0) | 4 (<1) | 6 (<1) |
| Unknown | 64 (5) | 11 (4) | 298 (4) | 373 (4) |
| Other | 27 (2) | 11 (4) | 120 (2) | 158 (2) |
| Ethnicity | ||||
| Hispanic or Latino | 63 (5) | 7 (3) | 278 (4) | 348 (4) |
| Not Hispanic or Latino | 946 (70) | 142 (52) | 5531 (72) | 6619 (71) |
| Unknown | 333 (25) | 123 (45) | 1902 (25) | 2358 (25) |
| Region | 301 (22) | 24 (9) | 795 (10) | 1120 (12) |
| US | 301 (22) | 24 (9) | 795 (10) | 1120 (12) |
| RoW | 1041 (78) | 248 (91) | 6916 (90) | 8205 (88) |
| Trial | ||||
| 1 | 0 (0) | 11 (4) | 918 (12) | 929 (10) |
| 2 | 0 (0) | 18 (7) | 460 (6) | 478 (5) |
| 3 | 45 (3) | 11 (4) | 123 (2) | 179 (2) |
| 4 | 33 (2) | 6 (2) | 103 (1) | 142 (2) |
| 5 | 125 (9) | 16 (6) | 553 (7) | 694 (7) |
| 6 | 99 (7) | 30 (11) | 468 (6) | 597 (6) |
| 7 | 181 (13) | 11 (4) | 696 (9) | 888 (10) |
| 8 | 215 (16) | 15 (6) | 722 (9) | 952 (10) |
| 9 | 0 (0) | 14 (5) | 708 (9) | 722 (8) |
| 10 | 115 (9) | 24 (9) | 622 (8) | 761 (8) |
| 11 | 40 (3) | 0 (0) | 117 (2) | 157 (2) |
| 12 | 139 (10) | 4 (1) | 451 (6) | 594 (6) |
| 13 | 140 (10) | 0 (0) | 646 (8) | 786 (8) |
| 14 | 46 (3) | 12 (4) | 114 (1) | 172 (2) |
| 15 | 0 (0) | 86 (32) | 232 (3) | 318 (3) |
| 16 | 164 (12) | 14 (5) | 778 (10) | 956 (10) |
RoW, rest of world.
Population in MM clinical trials based on race. The figure depicts the percentage of patients screened, enrolled, or excluded based on their race. Enrolled patients included eligible patients and those who were ineligible but enrolled as those with protocol violations. Percentages are based on the total denominator for each group.
Population in MM clinical trials based on race. The figure depicts the percentage of patients screened, enrolled, or excluded based on their race. Enrolled patients included eligible patients and those who were ineligible but enrolled as those with protocol violations. Percentages are based on the total denominator for each group.
Population in MM clinical trials based on ethnicity. The figure depicts the percentage of patients screened, enrolled, or excluded based on their ethnicity. Enrolled patients included eligible patients and those who were ineligible but enrolled as those with protocol violations. Percentages are based on the total denominator for each group.
Population in MM clinical trials based on ethnicity. The figure depicts the percentage of patients screened, enrolled, or excluded based on their ethnicity. Enrolled patients included eligible patients and those who were ineligible but enrolled as those with protocol violations. Percentages are based on the total denominator for each group.
Of the 9325 patients who were screened, 17% (n = 1614; range 2%-16%) were categorized as ineligible (Table 1). Of the 1614 patients, 14% were not enrolled and identified as excluded, and 3% were enrolled and identified as having protocol violations. Ineligibility rates were higher among Black patients (24%) and racial subgroups classified as other (23%) than among White patients (17%). Numerically higher rates of Black patients, other, and unknown racial subgroups compared with White patients were excluded, but the difference was <5%. The Asian racial subgroup had the lowest trial ineligibility and exclusion rate among the racial subgroups (11%). The ineligibility and excluded rates were not different based on ethnicity (Table 2).
Trial patients by race and ethnicity
| Total screened . | White N = 7787 N (%) . | Black N = 362 N (%) . | Asian N = 629 N (%) . | Other∗ N = 174 N (%) . | Unknown race N = 373 N (%) . | Hispanic or Latino N = 348 N (%) . | Not Hispanic or Latino N = 6619 N (%) . | Unknown ethnicity N = 2358 N (%) . |
|---|---|---|---|---|---|---|---|---|
| Ineligible | 1338 (17) | 88 (24) | 73 (12) | 40 (23) | 75 (20) | 70 (20) | 1088 (16) | 456 (19) |
| Excluded | 1118 (14) | 60 (17) | 71 (11) | 29 (17) | 64 (17) | 63 (18) | 946 (14) | 333 (14) |
| Total screened . | White N = 7787 N (%) . | Black N = 362 N (%) . | Asian N = 629 N (%) . | Other∗ N = 174 N (%) . | Unknown race N = 373 N (%) . | Hispanic or Latino N = 348 N (%) . | Not Hispanic or Latino N = 6619 N (%) . | Unknown ethnicity N = 2358 N (%) . |
|---|---|---|---|---|---|---|---|---|
| Ineligible | 1338 (17) | 88 (24) | 73 (12) | 40 (23) | 75 (20) | 70 (20) | 1088 (16) | 456 (19) |
| Excluded | 1118 (14) | 60 (17) | 71 (11) | 29 (17) | 64 (17) | 63 (18) | 946 (14) | 333 (14) |
Ineligibility compared with screened population by race and ethnicity.
Other includes 2 American Indian and Alaskan Native patients.
The reasons for trial ineligibility for the total analysis population and for each race category are displayed in Table 3. Among the total population screened in the 16 trials, failure to meet disease-related criteria (25%) was the most common reason for ineligibility. Failure to meet protocol-specific hematologic laboratory criteria (19%), followed by failure to meet treatment-related criteria (18%), was the most common reason for ineligibility among Black patients. Failure to meet disease-related criteria was the most common reason for ineligibility among White (28%) and Asian (29%) patients. Disease-related criteria was the most common reason for trial ineligibility in both Hispanic (21%) and non-Hispanic (27%) patients.
Ineligibility criteria by race and ethnicity
| Reasons for ineligibility∗ . | Total N = 1614 n (%) . | Race . | Ethnicity . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| White N = 1338 n (%) . | Black N = 88 n (%) . | Asian N = 73 n (%) . | Other∗ N = 40 n (%) . | Unknown N = 75 n (%) . | Hispanic or Latino N = 70 n (%) . | Not Hispanic or Latino N = 1088 n (%) . | Unknown N = 456 n (%) . | ||
| Disease related | 431 (27) | 368 (28) | 16 (18) | 21 (29) | 9 (22) | 17 (23) | 15 (21) | 291 (27) | 125 (27) |
| Failure to meet treatment related criteria | 195 (12) | 167 (12) | 15 (17) | 4 (5) | 3 (8) | 6 (8) | 7 (10) | 136 (12) | 52 (11) |
| Received restricted investigational drugs or chemotherapy within time period | 75 (5) | 63 (5) | 5 (6) | 2 (3) | 1 (2) | 4 (5) | 6 (9) | 40 (4) | 29 (6) |
| Hepatic function | 12 (1) | 10 (1) | NA | 1 (1) | 1 (2) | NA | NA | 6 (1) | 6 (1) |
| Renal function | 52 (3) | 44 (3) | 4 (5) | 1 (1) | 1 (2) | 2 (3) | NA | 39 (4) | 13 (3) |
| Cardiac function | 73 (5) | 63 (5) | 3 (3) | 4 (5) | 1 (2) | 2 (3) | 3 (4) | 42 (4) | 28 (6) |
| Pulmonary function | 37 (2) | 33 (2) | NA | 2 (3) | NA | 2 (3) | 4 (6) | 31 (3) | 2 (<1) |
| Hematology laboratory criteria | 171 (11) | 132 (10) | 17 (19) | 8 (11) | 6 (15) | 8 (11) | 2 (3) | 76 (7) | 93 (20) |
| Unspecified laboratory criteria† | 248 (15) | 197 (15) | 8 (9) | 14 (19) | 5 (12) | 24 (32) | 10 (14) | 162 (15) | 76 (17) |
| Active infection | 25 (2) | 21 (2) | 1 (1) | NA | 3 (8) | NA | NA | 15 (1) | 10 (2) |
| HIV or hepatitis virus | 31 (2) | 27 (2) | 3 (3) | 1 (1) | NA | NA | 1 (1) | 20 (2) | 10 (2) |
| Preexisting neuropathy | 7 (<1) | 6 (0) | 1 (1) | NA | NA | NA | NA | 5 (0) | 2 (<1) |
| Prior malignancy | 31 (2) | 26 (2) | 1 (1) | 2 (3) | 2 (5) | NA | 1 (1) | 22 (2) | 8 (2) |
| Contraception related | 67 (4) | 58 (4) | 3 (3) | 4 (5) | 1 (2) | 1 (1) | NA | 5 (0) | 62 (14) |
| Other IE exclusions | 274 (17) | 228 (17) | 18 (20) | 12 (16) | 12 (30) | 4 (5) | 8 (11) | 109 (10) | 157 (34) |
| Patient decision | 81 (5) | 69 (5) | 6 (7) | 3 (4) | 1 (2) | 2 (3) | 6 (9) | 68 (6) | 7 (2) |
| Missing | 84 (5) | 63 (5) | 3 (3) | 10 (14) | 2 (5) | 6 (8) | 9 (13) | 62 (6) | 13 (3) |
| Reasons for ineligibility∗ . | Total N = 1614 n (%) . | Race . | Ethnicity . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| White N = 1338 n (%) . | Black N = 88 n (%) . | Asian N = 73 n (%) . | Other∗ N = 40 n (%) . | Unknown N = 75 n (%) . | Hispanic or Latino N = 70 n (%) . | Not Hispanic or Latino N = 1088 n (%) . | Unknown N = 456 n (%) . | ||
| Disease related | 431 (27) | 368 (28) | 16 (18) | 21 (29) | 9 (22) | 17 (23) | 15 (21) | 291 (27) | 125 (27) |
| Failure to meet treatment related criteria | 195 (12) | 167 (12) | 15 (17) | 4 (5) | 3 (8) | 6 (8) | 7 (10) | 136 (12) | 52 (11) |
| Received restricted investigational drugs or chemotherapy within time period | 75 (5) | 63 (5) | 5 (6) | 2 (3) | 1 (2) | 4 (5) | 6 (9) | 40 (4) | 29 (6) |
| Hepatic function | 12 (1) | 10 (1) | NA | 1 (1) | 1 (2) | NA | NA | 6 (1) | 6 (1) |
| Renal function | 52 (3) | 44 (3) | 4 (5) | 1 (1) | 1 (2) | 2 (3) | NA | 39 (4) | 13 (3) |
| Cardiac function | 73 (5) | 63 (5) | 3 (3) | 4 (5) | 1 (2) | 2 (3) | 3 (4) | 42 (4) | 28 (6) |
| Pulmonary function | 37 (2) | 33 (2) | NA | 2 (3) | NA | 2 (3) | 4 (6) | 31 (3) | 2 (<1) |
| Hematology laboratory criteria | 171 (11) | 132 (10) | 17 (19) | 8 (11) | 6 (15) | 8 (11) | 2 (3) | 76 (7) | 93 (20) |
| Unspecified laboratory criteria† | 248 (15) | 197 (15) | 8 (9) | 14 (19) | 5 (12) | 24 (32) | 10 (14) | 162 (15) | 76 (17) |
| Active infection | 25 (2) | 21 (2) | 1 (1) | NA | 3 (8) | NA | NA | 15 (1) | 10 (2) |
| HIV or hepatitis virus | 31 (2) | 27 (2) | 3 (3) | 1 (1) | NA | NA | 1 (1) | 20 (2) | 10 (2) |
| Preexisting neuropathy | 7 (<1) | 6 (0) | 1 (1) | NA | NA | NA | NA | 5 (0) | 2 (<1) |
| Prior malignancy | 31 (2) | 26 (2) | 1 (1) | 2 (3) | 2 (5) | NA | 1 (1) | 22 (2) | 8 (2) |
| Contraception related | 67 (4) | 58 (4) | 3 (3) | 4 (5) | 1 (2) | 1 (1) | NA | 5 (0) | 62 (14) |
| Other IE exclusions | 274 (17) | 228 (17) | 18 (20) | 12 (16) | 12 (30) | 4 (5) | 8 (11) | 109 (10) | 157 (34) |
| Patient decision | 81 (5) | 69 (5) | 6 (7) | 3 (4) | 1 (2) | 2 (3) | 6 (9) | 68 (6) | 7 (2) |
| Missing | 84 (5) | 63 (5) | 3 (3) | 10 (14) | 2 (5) | 6 (8) | 9 (13) | 62 (6) | 13 (3) |
A patient may contribute to more than 1 reason for screen failure.
IE, inclusion/exclusion.
Other includes 2 American Indian and Alaskan Native patients.
Includes combined hematologic, renal, and hepatic function criteria.
Comparing the reasons for trial ineligibility for Black patients vs the White subgroup, a higher percentage of Black patients failed to meet treatment-related (17%) and hematology laboratory-related (19%) eligibility criteria than White patients (12% and 10%, respectively). The rate of ineligibility in the unspecified category by the laboratory was lower among Black patients (9%) than among White patients (15%).
An exploratory analysis of the eligibility criteria of the enrolled patients, despite being ineligible, was conducted. Among the 272 patients who were enrolled for protocol violations, Black patients were most likely to be enrolled despite failing to meet the protocol-specific hematology laboratory criteria (29%) and unspecified laboratory criteria (21%), compared with 13% and 15% for White patients (Table 4). Other racial and ethnic subgroup results have not been reported because of the very small number of patients enrolled as protocol violations.
Reasons enrolled as protocol deviations
| Reasons for ineligibility . | Total N = 272 n (%) . | White N = 220 n (%) . | Black N = 28 n (%) . | Asian N = 2 n (%) . | Other N = 11 n (%) . | Unknown N = 11 n (%) . |
|---|---|---|---|---|---|---|
| Disease related | 41 (15) | 35 (16) | 2 (7) | 0 | 4 (36) | NA |
| Failure to meet treatment related criteria | 44 (16) | 37 (17) | 3 (11) | 1 (50) | 1 (9) | 2 (18) |
| Received restricted investigational drugs or chemotherapy within time period | 29 (11) | 22 (10) | 5 (18) | 0 | 0 | 2 (18) |
| Hepatic function | 5 (2) | 5 (2) | 0 | 0 | 0 | 0 |
| Renal function | 11 (4) | 7 (3) | 3 (11) | 0 | 0 | 1 (9) |
| Cardiac function | 16 (6) | 14 (6) | 0 | 0 | 0 | 2 (18) |
| Pulmonary function | 11 (4) | 11 (5) | 0 | 0 | 0 | 0 |
| Hematology laboratory criteria | 37 (14) | 29 (13) | 8 (29) | 0 | 0 | 0 |
| Unspecified laboratory criteria | 45 (17) | 33 (15) | 6 (21) | 1 (50) | 1 (9) | 4 (36) |
| Active infection | 17 (6) | 14 (6) | 1 (4) | 0 | 2 (18) | 0 |
| HIV or hepatitis virus | 7 (3) | 7 (3) | 0 | 0 | 0 | 0 |
| Pre-existing neuropathy | 2 (1) | 2 (1) | 0 | 0 | 0 | 0 |
| Prior malignancy | 9 (3) | 6 (3) | 1 (4) | 0 | 2 (18) | 0 |
| Contraception related | 1 (<1) | 1 (<1) | 0 | 0 | 0 | 0 |
| Other IE exclusions | 14 (5) | 11 (5) | 2 (7) | 0 | 1 (9) | 0 |
| Reasons for ineligibility . | Total N = 272 n (%) . | White N = 220 n (%) . | Black N = 28 n (%) . | Asian N = 2 n (%) . | Other N = 11 n (%) . | Unknown N = 11 n (%) . |
|---|---|---|---|---|---|---|
| Disease related | 41 (15) | 35 (16) | 2 (7) | 0 | 4 (36) | NA |
| Failure to meet treatment related criteria | 44 (16) | 37 (17) | 3 (11) | 1 (50) | 1 (9) | 2 (18) |
| Received restricted investigational drugs or chemotherapy within time period | 29 (11) | 22 (10) | 5 (18) | 0 | 0 | 2 (18) |
| Hepatic function | 5 (2) | 5 (2) | 0 | 0 | 0 | 0 |
| Renal function | 11 (4) | 7 (3) | 3 (11) | 0 | 0 | 1 (9) |
| Cardiac function | 16 (6) | 14 (6) | 0 | 0 | 0 | 2 (18) |
| Pulmonary function | 11 (4) | 11 (5) | 0 | 0 | 0 | 0 |
| Hematology laboratory criteria | 37 (14) | 29 (13) | 8 (29) | 0 | 0 | 0 |
| Unspecified laboratory criteria | 45 (17) | 33 (15) | 6 (21) | 1 (50) | 1 (9) | 4 (36) |
| Active infection | 17 (6) | 14 (6) | 1 (4) | 0 | 2 (18) | 0 |
| HIV or hepatitis virus | 7 (3) | 7 (3) | 0 | 0 | 0 | 0 |
| Pre-existing neuropathy | 2 (1) | 2 (1) | 0 | 0 | 0 | 0 |
| Prior malignancy | 9 (3) | 6 (3) | 1 (4) | 0 | 2 (18) | 0 |
| Contraception related | 1 (<1) | 1 (<1) | 0 | 0 | 0 | 0 |
| Other IE exclusions | 14 (5) | 11 (5) | 2 (7) | 0 | 1 (9) | 0 |
IE, inclusion/exclusion.
Discussion
Underrepresentation of racial and ethnic groups in cancer clinical trials has been reported previously in multiple studies analyzing racial and ethnic representation in FDA approvals in oncology.20-22
In our analysis, consistent with previously reported analyses, screening for trial eligibility was substantially lower across all racial and ethnic minority subgroups compared with White patients. Although the incidence of MM in African American people is twice that of White people in the US patient population, only 4% of the patients screened for trial eligibility in our pooled analysis were Black, whereas 84% were White.
Based on our review, this is the first study to evaluate specific trial eligibility criteria as a potential barrier to enrollment of underrepresented racial and ethnic subgroups in MM clinical trials.
Ineligibility rates were higher among Black patients than among other racial subgroups. Racial differences were noted in the specific eligibility criteria. Black patients were more likely to be ineligible for a trial because of failure to meet the hematology laboratory criteria and protocol-specified treatment-related criteria. The higher rates of ineligibility due to hematologic laboratory criteria and failure to meet treatment-related criteria for Black patients are not surprising. Evidence from the literature suggests that racial differences exist in neutrophil counts (benign neutropenia), and the threshold for normal neutrophil counts may be lower in Black people than in White people.23 Rates of anemia have also been reported to be higher in African American patients with MM than in White patients.24 Broad exclusion of patients, primarily based on absolute values of hematology lab tests, may contribute to the underrepresentation of Black patients in MM clinical trials.
Prior studies in patient populations with MM have identified differences in access and receipt of therapies by race and ethnicity8,25 In a study that evaluated 639 patients with newly diagnosed MM from the Multiple Myeloma Research Foundation CoMMpass registry, African American patients were less likely to receive standard triplet therapies (alkylator, proteasome, or immunomodulatory based) and first-line autologous stem cell transplant compared with White patients.26 Clinical trials designed to support regulatory approval of an indication in a particular patient population often include eligibility criteria requiring a minimum number of prior lines of therapy or receipt of specific therapies. Because Black patients might not have received the specified treatments for enrollment, a delay in receipt or lack of receipt of a standard of care therapy, such as that described in the Multiple Myeloma Research Foundation CoMMpass registry study, can result in ineligibility and increase racial disparities in clinical trial enrollment, which in turn reduces the evidence to offer new therapies to racial and ethnic minority groups, propagating a cycle.
Stringent eligibility criteria requirements for normal or adequate organ function (eg, renal, hepatic, and cardiac) and minimal comorbidities have previously been reported as a potential barrier to enrollment of racial and ethnic minority groups in cancer clinical trials. In a single-center study evaluating 235 African American individuals who were consecutively diagnosed with cancer, 17% were ineligible for treatment trials because of presence of comorbidities. However, this study did not report on other races or ethnicity.16 In the National Cancer Institute’s Community Cancer Center Program, a higher proportion of non-Hispanic Black individuals evaluated for breast, colorectal, and genitourinary trials did not meet the eligibility criteria for enrollment due to comorbidities compared with the other racial groups.17 In our study, except for the hematology laboratory criteria, screen failure reasons for other organ function criteria (renal and hepatic function) and comorbid conditions (ie, cardiac and pulmonary function, active infection, hepatitis, and HIV) did not differ among the racial groups; however, the data were limited by the small number of patients within each racial group other than White people.
A unique aspect of our study is the analysis of patients who were enrolled despite meeting the eligibility criteria. Notably, although 24% of Black patients were initially assessed as ineligible, 17% were ultimately excluded from trial enrollment. Black patients assessed as ineligible due to hematologic laboratory criteria were more likely to be enrolled for protocol violations. This finding suggests that physicians may be factoring in racial and ethnic differences in hematologic laboratory values when considering these patients for inclusion in trials. Although this is encouraging, it relies on individual investigator discretion and may not be sufficient to eliminate racial disparities in enrollment. Clinical trial eligibility criteria should likely consider potential variations in laboratory values that occur because of variations in race or ethnicity and consider broadening the eligibility criteria for hematologic function.27
Limitations
Several limitations of this study should be considered when interpreting the results. There was a lack of granularity regarding failure to meet hematologic eligibility criteria, for example, limiting the ability to discern whether a patient was ineligible due to not meeting a minimum neutrophil count level vs a platelet level. Some trial data sets only captured ineligibility due to broad laboratory criteria and did not separately capture hematologic laboratory criteria from renal and hepatic laboratory criteria. This lack of granularity may have contributed to the lack of racial differences in ineligibility based on the organ function criteria. There were few patients enrolled with Hispanic ethnicity and a high percentage of patients of unknown ethnicity, which limited the ability to analyze differences in eligibility criteria by ethnicity. Furthermore, patient race and ethnicity are captured in clinical trial CRFs, which may not be sufficiently designed to capture multiracial or multiethnic categories. Despite pooling data from 16 registrational trials that supported the approval of new therapies or new indications of MM, the number of patients in racial and ethnic subgroups screened for trial enrollment was very low; eg, <5% of Black and Hispanic patients with MM were screened. Hence, a robust statistical analysis for reasons for screen failure could not be conducted and the results are descriptive in nature and should be considered hypothesis generating. The small numbers also precluded the assessment of potential differences in reasons for ineligibility based on lines of therapy and region.
Conclusions
Our analysis of MM clinical trials suggests that certain protocol-specific eligibility criteria may contribute to racial and ethnic disparities in enrollment to MM clinical trials. The FDA has several ongoing initiatives and has published several guidance documents with specific strategies to enhance diversity within clinical trials and to increase the representation of traditionally underrepresented patient populations in cancer clinical trials.28 To facilitate the inclusion of patient populations that will ultimately receive cancer therapies, the FDA has also provided guidance to broaden eligibility criteria in oncology clinical trials.29 Designing cancer clinical trials that include patients with organ dysfunction and prior or concurrent malignancies, including this information in the labeling, can facilitate the safe and effective use of these products across a broader patient population, likely to use the drug in clinical practice. In addition, investigators should consider racial differences in hematologic laboratory values when defining the eligibility criteria in cancer clinical trials.
However, although trial-level eligibility criteria could be a potential barrier to the enrollment of patients when looking at the rates of patients who were enrolled despite initially failing to meet the eligibility criteria in our study, the rates were similar across racial subgroups. The proportion of ineligible Black patients who were ultimately enrolled was higher than the proportion of White/Other patients that were ineligible but enrolled. This observation, along with the low screening rates for the underrepresented racial subgroups in our study, indicates that other factors, including individual patient-level barriers and system-level barriers, may influence trial enrollment.30 Given the multiple factors that may contribute to the underrepresentation of racial and ethnic groups in clinical trials, broad stakeholder collaboration to address other factors, in addition to broadening eligibility criteria, is needed to address disparities. A recent FDA viewpoint paper31 outlining a framework for increasing diversity in clinical trials is an important step in this direction and potentially facilitates future enrollment strategies for underrepresented racial and ethnic subgroups. More recently, the FDA published a draft guidance recommending a similar framework.32 The FDA guidance recommends sponsors developing medical products to prospectively define a plan that includes but is not limited to enrollment goals for underrepresented racial and ethnic study populations and study design features that will allow for an assessment of safety and effectiveness during the entire clinical development of the product.
Authorship
Contribution: B.K., L.L.F., A.B., R.E., V.B., E.P., T.G., M.R.T., R.P., L.F.-A., and N.G. collected the data and wrote and reviewed the manuscript.
Conflict-of-interest disclosure: L.L.F. is an employee of Cota Health care Inc and owns stock in the company.The remaining authors declare no competing financial interests.
The current affiliation for L.L.F. is Cota Healthcare, New York, NY.
The current affiliation for T.G. is T Gwise Consulting LLC, St. Petersburg, FL.
Correspondence: Bindu Kanapuru, Center for Drug Evaluation and Research, US Food and Drug Administration, 10903 New Hampshire Ave, Silver Spring, MD 20993; e-mail: bindu.kanapuru@fda.hhs.gov.
References
Author notes
Contact the corresponding author for any data requests: Bindu Kanapuru (bindu.kanapuru@fda.hhs.gov).
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


Comments
From Duffy Null to Duffy Full: Clinical Trial Diversity Matters
Eight percent of Black patients assessed as ineligible by hematological criteria were enrolled on trials1, likely reflecting physician understanding of Duffy-null associated neutrophil counts (DANC). Individual investigator discretion and sponsor eligibility exceptions should be superseded by dedicated testing for Duffy status and specific protocol guidance. Lowering eligibility neutrophil thresholds for Duffy-null individuals to no higher than 1000 cells/uL will minimize unnecessary exclusion. Beyond eligibility criteria, dose adjustment tables also require careful consideration, potentially with different thresholds for Duffy-null participants. There is a major gap in evidence to guide the careful balance between safety and efficacy with dosing of myelosuppressive mediations in this population. For example, azathioprine is more frequently discontinued Duffy-null patients, but the optimal dosing strategy is unclear5. Mindfully including Duffy-null individuals in clinical trials will enhance diversity of research participants and enable understanding of optimal dosing for this large global population.
1. Kanapuru B, Fernandes L, Baines AC, et al. Eligibility criteria and Enrollment of a Diverse Racial and Ethnic population in Multiple Myeloma Clinical Trials. Blood. 2023.
2. Howes RE, Patil AP, Piel FB, et al. The global distribution of the Duffy blood group. Nat Commun. 2011;2:266.
3. Merz LE, Story CM, Osei MA, et al. Absolute Neutrophil Count by Duffy Status Among Healthy Black and African American Adults. Blood Advances. 2022:bloodadvances.2022007679.
4. Hsieh MM, Tisdale JF, Rodgers GP, Young NS, Trimble EL, Little RF. Neutrophil count in African Americans: lowering the target cutoff to initiate or resume chemotherapy? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(10):1633-1637.
5. Dickson AL, Daniel LL, Jackson E, et al. Race, Genotype, and Azathioprine Discontinuation : A Cohort Study. Ann Intern Med. 2022;175(8):1092-1099.