In this issue of Blood, Kim et al1 from the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) take us one giant leap forward in our understanding of adult KMT2A-rearranged (KMT2A-r) B-cell precursor acute lymphoblastic leukemia (B-ALL). For the first time, they demonstrate the ability to risk stratify young adults within this high-risk group and identify patients with KMT2A-r B-ALL with outstanding outcomes when treated with intensive, pediatric-inspired chemotherapy, even without allogeneic hematopoietic stem cell transplant (HSCT).
We have come a long way since 1948 when Sidney Farber announced transient responses in 5 children treated with the folic acid antagonist aminopterin.2 What has followed is 75 years of worldwide, pediatrician-led collaboration to improve outcomes in ALL. We are fortunate that now >90% of children diagnosed with ALL in resourced settings are cured with modern, risk-adapted chemotherapy.3 Younger adults treated with intensive, pediatric-style asparaginase-based chemotherapy are also now frequently cured, almost (although not quite) as often as children.4 The use of HSCT is typically reserved for high-risk patients (by various definitions) or those not achieving an optimal early response.5
Still, KMT2A-r ALL remains a feared subtype of ALL with historically dismal outcomes in infants,6 as well as adults.7 Indeed, relapsed KMT2A-r ALL is a dreaded occurrence given infrequent and fleeting responses to available immunotherapy (blinatumomab, inotuzumab ozogamicin, and chimeric antigen receptor T cells) and risk of lineage switch. Thus, most adult groups recommend HSCT in first complete remission (CR1) for eligible patients based on the presumption of consistently adverse disease biology. Until now, there have been no data to help a clinician further risk stratify a patient or support a non-HSCT approach.
In this context, the GRAALL investigators studied 141 adult patients with KMT2A-r ALL (median age, 41 years; range, 18-59 years; 12.9% of overall GRAALL cohort) treated on 3 successive GRAALL trials of intensive pediatric-inspired chemotherapy (GRAALL-2003, GRAALL-2005, and GRAALL-2014) with the goal of identifying factors associated with clinical outcomes. The KMT2A-r patients in the GRAALL cohort were, as expected, characterized by high-risk clinical features (older age and higher white blood cell count) but almost universally achieved CR1 with a single induction with a 5-year cumulative incidence of relapse (CIR) of 40.7% and a 5-year overall survival (OS) of 53.3%. The authors then demonstrated that patients with KMT2A-r ALL could be risk stratified using genetic profiling and measurable residual disease (MRD) assessment.
Regarding genetics, like infant KMT2A-r ALL, there was a low rate of additional mutations, with the most frequent being subclonal mutations in RAS and receptor tyrosine kinase genes (38%). Less frequent, but notably prognostic, were mutations in TP53 (14%) and IKZF1 deletions (8%). Patients with any of these high-risk mutations had a higher 5-year CIR (69.3% vs 36.2%; P = .001) and a lower 5-year OS (28.1% vs 60.7%; P = .006). The statistics were most robust for the more-frequent TP53-mutated patients, all of whom had aggressive disease biology (ie, relapsing within 6 months). Regarding MRD assessment, monitoring using a KMT2A genomic fusion assay, rather than tracking Ig/T-cell receptor rearrangements, was more reliable (similar to infant KMT2A-r ALL), as the latter are either absent at diagnosis or at risk for clonal evolution. Remarkably, patients with early KMT2A-based MRD response (of note, all lacking the aforementioned high-risk genetics at diagnosis) had superb outcomes (3-year CIR, 7.1%; and OS, 92.9%).
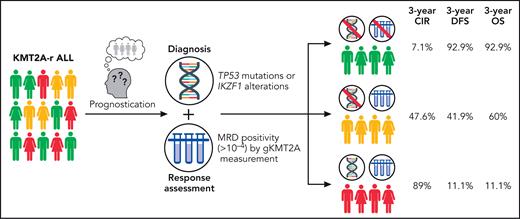
Translating their findings onto a clinically useful paradigm, the GRAALL investigators created a classifier combining molecular profiling at diagnosis (if no high-risk mutations: Onco−; if high-risk mutations: Onco+) and MRD to create 3 groups with distinct outcomes: Onco−/MRD−, Onco−/MRD+, and Onco+/MRD+, which were associated with starkly divergent 3-year CIR (7.1%, 47.6%, and 88.9%, respectively), disease-free survival (92.9%, 41.9%, and 11.1%, respectively), and OS (92.9%, 60.0%, and 11.1%, respectively) rates (see figure). Notably, the excellent findings of the MRD− group occurred in the absence of HSCT in CR1, as the GRAALL-14 trial did not allocate KMT2A-r patients to HSCT if they achieved MRD− CR.
Prognostication model for KMT2A-rearranged (KMT2A-r) acute lymphoblastic leukemia (ALL). CIR, cumulative incidence of relapse; DFS, disease-free survival; gKMT2A, genomic fusion assay for KMT2A; MRD, measurable residual disease; OS, overall survival. Professional illustration by Patrick Lane, ScEYEnce Studios.
Prognostication model for KMT2A-rearranged (KMT2A-r) acute lymphoblastic leukemia (ALL). CIR, cumulative incidence of relapse; DFS, disease-free survival; gKMT2A, genomic fusion assay for KMT2A; MRD, measurable residual disease; OS, overall survival. Professional illustration by Patrick Lane, ScEYEnce Studios.
Thus, in the context of younger adult patients receiving intensive pediatric-type chemotherapy, the discovery of a KMT2A-r may not warrant automatic dismay. Rather, with risk stratification by molecular profiling at diagnosis and MRD monitoring, risk-adapted therapy appeared beneficial, akin to the dynamic approach employed for other subtypes of Philadelphia chromosome–negative ALL and Philadelphia chromosome–positive ALL.5 This promises to allow intensively treated adults with KMT2A-r ALL without mutant TP53 or IKZF1 deletion and an optimal response to avoid the toxicity of (typically total body irradiation-based) myeloablative HSCT.
So, what next? The classifier created by the GRAALL investigators will ideally be studied in additional large ALL cohorts, including among children and young adults treated with other intensive asparaginase-based pediatric regimens, as well as among older patients treated with non–asparaginase-based treatment with or without novel agents. More important, as novel treatment approaches for KMT2A-r ALL, including blinatumomab consolidation8 and menin inhibitors,9 are being developed with the potential to modify the outcomes of higher-risk patients, their effect according to the classifier risk groups will need to be studied. Finally, the ability of therapeutic intensification with HSCT to improve cure in higher-risk patients needs further evaluation. Given the rarity of KMT2A-r ALL (5%-10% of noninfant childhood and adult ALL), the leukemia community will need to cooperate in an international KMT2A-r program to speed ascertainment of data and clinical understanding as the therapeutic landscape rapidly evolves.
Philadelphia chromosome–positive B-ALL, once a similarly feared ALL subtype, has been transformed by better treatments as well as more precise monitoring and refined risk stratification schemes, changing from an adverse to a nonadverse subtype.10 Can we dare to imagine the same for KMT2A-r ALL? Basic, translational, and clinical research in ALL has taken us to the (metaphorical) moon over the past 75 years. Now, let’s go to Mars!
Conflict-of-interest disclosure: M.R.L. receives research support from AbbVie and Novartis; honoraria from Pfizer, Novartis, and Jazz Pharmaceuticals. S.S. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal