Hematopoietic stem cell transplantation has been the standard of care for infants with severe combined immunodeficiency (SCID) since 1968, and although the requirement for pretransplant cytoreductive chemotherapy to achieve durable reconstitution has been hotly debated, it is now clear that most SCID genotypes benefit from preparative chemotherapy. However, the requirement for preparative chemotherapy is less clear for interleukin-7 receptor α chain (IL-7Rα) deficiency. In this issue of Blood, Kaiser et al1 present data on the role of IL-7 signaling in humans that help to clarify this issue.
Patients with SCID have genetic defects that block development of T lymphocytes, and, depending on the specific defect, the development or function of B lymphocytes as well as natural killer cells may also be blocked.2 For many years, although hotly debated,3 infusion of hematopoietic stem cells was considered to be adequate to confer durable, diverse immunity, although it was acknowledged that B-lymphocyte immunity may not be reconstituted and patients may require lifelong immunoglobulin treatment.4 More recently, accumulating evidence suggests that the administration of pretransplant preparative chemotherapy facilitates the development of enduring thymopoiesis, as the thymus is continuously seeded by donor progenitors from the bone marrow.5 The debate has been complicated by the fact that response to infusion alone is dependent on the specific genotype. Thus, blocks in later T- and B-lymphocyte development, due to RAG1/2 or DLRE1C mutations, give rise to poor immune reconstitution with limited T-lymphocyte receptor diversity, poor thymopoiesis, and absent B lymphocytes, thought to be due to the fact that the osteomedullary and thymic niches are full of T- and B-lymphocyte precursors and there is no space for infused donor cells. However, genetic defects that interrupt early lymphocyte development, such as defects in IL-2Rγ, JAK3, or IL-7Rα, so-called “permissive” phenotypes, respond to infusion by the development of thymopoiesis and broad T-lymphocyte receptor diversity. Space in the thymic niche can be occupied by donor lymphocyte precursors enabling T-lymphocyte development, despite the osteomedullary niche being occupied by recipient precursor and mature B lymphocytes. In these defects, donor B lymphocytes generally do not develop with just infusion.6 The question of B-lymphocyte function has been more problematic in these early defects, even though recipient B lymphocytes are present. Patients with IL-2Rγ/JAK3 SCID often fail to make immunoglobulin G or vaccine humoral responses following infusion of donor stem cells, despite developing a broad repertoire of donor stem cell–derived naïve T lymphocytes. IL-21 is essential for human B-lymphocyte proliferation, immunoglobulin isotype switching, and antibody secretion and acts by binding a heterodimeric receptor comprising the IL-21 receptor (R) and the common γ chain that is also a component of the IL-2R. Recipient IL2Rγ/JAK3–deficient B lymphocytes lack the common γ chain component of both IL-2R and IL-21R and thus fail to initiate the humoral response.7 Trying to understand the problem in IL-7Rα deficiency has been difficult; mice that lack IL-7 signaling fail to develop T and B lymphocytes, suggesting that IL-7 is critical for B lymphopoiesis, whereas in humans with IL-7Rα deficiency, T lymphocytes fail to develop but B lymphocytes appear to develop normally.
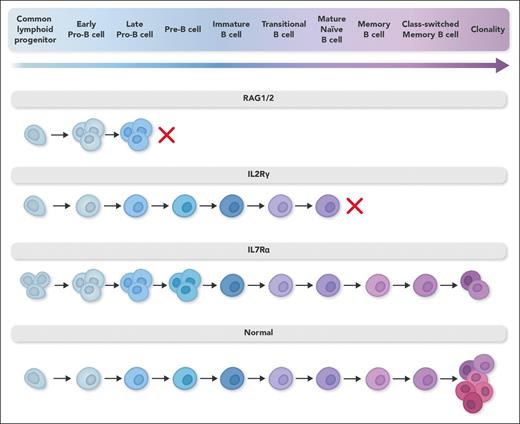
Following T-lymphocyte immune reconstitution after donor stem cell infusion, many, but not all, IL-7Rα–deficient patients with recipient B lymphocytes become independent of immunoglobulin replacement. Using a variety of methods, including detailed flow cytometric analysis and single-cell RNA sequencing of bone marrow samples from IL-7Rα–deficient patients and healthy controls and in vitro differentiation of B lymphocytes from umbilical cord blood and bone marrow from healthy donors, Kaiser et al have advanced our understanding of the role of IL-7 signaling in human B lymphopoiesis. They demonstrate that IL-7 signaling induces proliferation of early B-lymphocyte progenitors and increases expressions EBF1 and PAX5, which are required to stimulate B-lymphocyte specification and commitment of early lymphoid progenitors and has only a minor role in the prevention of apoptosis. They demonstrate that the clonality of the patients’ immunoglobulin heavy chain (IgH) repertoire was increased (see figure), likely because repertoire diversity is established from a significant and diverse early B-lymphocyte progenitor pool, expansion of which requires IL-7. The authors postulate that in patients, early progenitors underwent rapid IgH recombination associated with impaired proliferation in the absence of IL-7R signaling, thus creating a smaller pool of cells with diverse IgH alleles and restricting the final B-lymphocyte receptor repertoire. However, as the repertoire analysis was performed with naive mature B lymphocytes of age-matched healthy infants, and because patients with SCID are transplanted within the first year of life, it is not clear how a lack of IL-7 signaling would affect repertoire diversity at an older age in patients with SCID who received an unconditioned infusion.
B-lymphocyte differentiation in diverse severe combined immunodeficiency genotypes. B-lymphocyte differentiation pathway in different gene defects associated with severe combined immunodeficiency. In RAG1/2 defects, an early developmental block leads to proliferation of early precursors filling the osteomedullary niche and failure of B-lymphocyte development. In IL-2Rγ defects, recipient B lymphocytes develop normally, but failure of signaling through the common γ chain of the IL-21 receptor abrogates B-lymphocyte proliferation, immunoglobulin isotype switching, and antibody secretion. In IL-7Rα defects, recipient B-lymphocyte precursors do not differentiate as effectively as normal cells because of lack of signaling through the IL-7R, leading to a smaller pool of cells with diverse IgH alleles and restricted mature B-lymphocyte receptor diversity. Professional illustration by Somersault18:24.
B-lymphocyte differentiation in diverse severe combined immunodeficiency genotypes. B-lymphocyte differentiation pathway in different gene defects associated with severe combined immunodeficiency. In RAG1/2 defects, an early developmental block leads to proliferation of early precursors filling the osteomedullary niche and failure of B-lymphocyte development. In IL-2Rγ defects, recipient B lymphocytes develop normally, but failure of signaling through the common γ chain of the IL-21 receptor abrogates B-lymphocyte proliferation, immunoglobulin isotype switching, and antibody secretion. In IL-7Rα defects, recipient B-lymphocyte precursors do not differentiate as effectively as normal cells because of lack of signaling through the IL-7R, leading to a smaller pool of cells with diverse IgH alleles and restricted mature B-lymphocyte receptor diversity. Professional illustration by Somersault18:24.
Unfortunately, large cohort studies that examine the B-lymphocyte function of conditioned vs unconditioned patients are currently absent. The long-term effect of autologous, IL-7Rα–deficient early B-lymphocyte progenitors on later durable and diverse humoral immunity is unknown, and this question should now be urgently addressed.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal