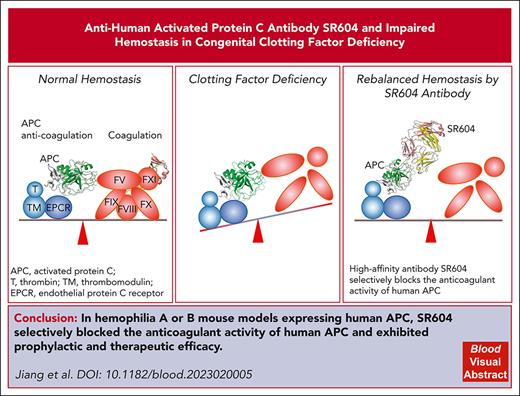
Key Points
We engineered a high-affinity antibody, SR604, which selectively blocks the anticoagulant activity of human APC.
SR604 exhibited prophylactic and therapeutic efficacy in mice with hemophilia A and B expressing human APC.
Abstract
Rebalance of coagulation and anticoagulation to achieve a hemostatic effect has recently gained attention as an alternative therapeutic strategy for hemophilia. We engineered a humanized chimeric antibody, SR604, based on a previously published murine antibody, HAPC1573, which selectively blocks the anticoagulant activity of human activated protein C (APC). SR604 effectively blocked the anticoagulation activities of APC in human plasma deficient in various coagulation factors in vitro with affinities ∼60 times greater than that of HAPC1573. SR604 exhibited prophylactic and therapeutic efficacy in the tail-bleeding and knee-injury models of hemophilia A and B mice expressing human APC (humanized hemophilic mice). SR604 did not interfere with the cytoprotection and endothelial barrier function of APC, nor were there obvious toxicity effects in humanized hemophilic mice. Pharmacokinetic study showed a high bioavailability (106%) of subcutaneously injected SR604 in cynomolgus monkeys. These results demonstrate that SR604 is expected to be a safe and effective therapeutic and/or prophylactic agent with a prolonged half-life for patients with congenital factor deficiencies including hemophilia A and B.
Introduction
Congenital factor deficiencies are a group of inherited bleeding disorders, which include deficiency of factor VIII (FVIII), FIX, FV, FVII, FX, or FXI.1-5 Among these genetic bleeding disorders, hemophilia A and hemophilia B, which are X-linked congenital deficiencies of FVIII or FIX, respectively, are the most common. Patients with severe hemophilia frequently develop bleeding in joints, muscles, and/or soft tissues either spontaneously or after injury, which often leads to disability or even death.6 The current standard treatment for hemophilia, especially in countries without adequate resources, is still factor replacement products. Most of these products must be IV injected 2 or 3 times every week. In addition, patients with either hemophilia A or B could develop inhibitory antibodies that neutralize the therapeutic effects of these products.7,8
To address the unmet need of hemophilia prophylactic treatment, nonfactor products for patients with hemophilia with or without inhibitors have been developed.5,9 The most successful example is hemlibra, a bispecific antibody to FIXa and FX.10,11 Although hemlibra represents a remarkable success, its efficacy is limited to patients with hemophilia A. Recently, rebalancing strategies based on inhibition of anticoagulant activities, including tissue factor pathway inhibitor12-14 or antithrombin III,15,16 have also been tested for patients with hemophilia A or B, with promising efficacy and safety profiles.
The protein C pathway is one of the most powerful anticoagulant mechanisms in blood vessels.17,18 When the blood vessel is injured, exposed tissue factor interacts with coagulation factors in the blood, such as activated FVII, which activates FX and initiates the extrinsic coagulation pathway. Activated FX together with activated FV activates prothrombin into thrombin, thereby promoting coagulation amplification. To counterbalance the coagulation amplification, thrombin binds to thrombomodulin on the endothelial cell surface to form the thrombin-thrombomodulin complex that converts protein C into activated protein C (APC). APC together with protein S inactivates activated FVIII and FV, thus negatively downregulating coagulation. These well-established anticoagulant mechanisms have recently been targeted to develop a new class of hemophilia therapies.5,19,20
Beyond its anticoagulant role, APC has cytoprotective and anti-inflammatory functions.21-24 We, and an independent group, previously reported a murine monoclonal antibody (mAb) HAPC1573 that specifically blocks the anticoagulant activity but not the cytoprotective and anti-inflammatory functions of APC.5,25 These results show that HAPC1573 mAb improves clotting defects in vitro in human plasma deficient of various coagulation factors as well as in vivo in both monkey and mouse hemophilia models. However, HAPC1573 affinity toward APC is ∼10 nM, which is much higher than the APC concentration (∼40 pM) in human plasma.26 In our previous study, we found that even a 5 mg/kg dose of HAPC1573 was insufficient to fully restore the hemostasis in the tail-bleeding mouse model of hemophilia.5 The high doses of HAPC1573 used in animal models limits further clinical studies.5,25
To develop a therapeutic candidate for clinical studies, we first engineered humanized HAPC1573 chimeric antibody, Humab-14, and then performed an affinity maturation study on Humab-14, which resulted in a new humanized HAPC1573 variant, SR604. SR604 has enhanced affinity with 4 point mutations in the complementarity-determining region (CDR) of HAPC1573. We found that this therapeutic candidate, SR604, effectively blocks the anticoagulation activities of APC in human coagulation factor–deficient plasma in vitro and in the tail-bleeding and knee-injury models in both hemophilia A and B mice, with affinities ∼60-times greater than that of HAPC1573 used in previous studies.
Materials and methods
Mice
Hemophilia A or B mouse models expressing human protein C (PROC+/+;F8−/− and PROC+/+;F9−/−) were generated by crossing transgenic mice expressing human protein C (PROC+/+) with either F8−/− or F9−/− mice (The Jackson Laboratory, Bar Harbor, ME). The generation of the PROC+/+mice was previously described.5 All genotype selections were via polymerase chain reaction. Mice were bred and maintained in specific-pathogen–free conditions in the Laboratory Animal Experimental Center at Soochow University. Mouse studies were approved by the animal use committee of the First Affiliated Hospital of Soochow University.
Antibody-binding kinetics analysis via surface plasmon resonance
All experiments were performed with a Biacore T200 instrument at 25°C, with running buffer HBS-EP (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4; 150 mM NaCl; 3 mM EDTA; and 0.05% surfactant P20). Briefly, mAbs were captured using antihuman immunoglobulin G (IgG) Fc antibody covalently immobilized via amine coupling to the surface of a CM5 sensor chip. The APC was injected at 7 concentrations ranging from 0.5 and 32 nM in twofold dilutions with the running buffer, resulting in concentrations of 0.5, 1, 2, 4, 8, 16, and 32 nM. The 8 nM concentration was run in duplicate. The chip surface with captured antibody was regenerated after each antigen injection with 10 mM glycine-hydrochloride, pH 1.5. Data obtained were via triplicate analyses and fit to a kinetics ratio of 1:1.
Tail-bleeding assay
PROC+/+;F8−/− and PROC+/+;F9−/− mice (male; aged 8-10 weeks; average body weight, 30 g) were treated with phosphate-buffered saline or SR604 antibody (0.025, 0.05, 0.1, or 0.2 mg/kg) via subcutaneous injection. After 24 hours, the mice were anesthetized with 1% sodium pentobarbital solution and then placed in the prone position. The 2-mm distal portion of the tails were removed with a scalpel, and the remainder of the tails were immediately immersed in 15-mL centrifuge tubes containing 0.9% saline that was preheated to 37°C in a water bath. The tails were kept vertical and below the body level. Each animal was monitored for 20 minutes. Blood loss was determined based on the hemoglobin content after red blood cell lysis with 2% acetic acid and was expressed in microliters of blood per gram of mouse body weight, assuming a hematocrit of 46%. If bleeding did not stop within 20 minutes, the experiment was terminated at 20 minutes, per the approved animal protocol, to avoid death during the procedure. For positive controls, 15 minutes before tail cutting, another group of PROC+/+;F8−/− mice were injected IV into the circulating blood with human plasma–derived FVIII (1 IU per mouse), and PROC+/+;F9−/− mice were injected with human plasma–derived prothrombin complex concentrate (PCC; 1 IU per mouse).
Knee-joint bleeding via needle puncture
Intra-articular bleeding was induced in the knee joint with a needle puncture following published methods.27 Briefly, the right knee capsule of anesthetized mice was punctured below the patella with a 30 × 0.5 G needle. Knee diameter before the needle injury and every other day until the 14th day after injury was measured with an electronic caliper, and the change of joint diameter was calculated as the percentage of the preinjury value. The uninjured left knee of the same mouse was used as control.
Inflammation induction and mouse survival study
Eight-week-old male PROC+/+;F8−/− and PROC+/+;F9−/− mice were used in this study (average weight, 30 g). SR604 (0.5 mg/kg) or HPC4 antibody (0.5 mg/kg, #01774, Genescript Biotech Corp) was administered through retro-orbital injection. Mice were then injected intraperitoneally with a sublethal dose of lipopolysaccharide (LPS; 10 mg/kg) and examined for survival every 12 hours for 3 days.
Statistics
Unless specified, values are expressed as means ± standard error of the mean. Survival curves were plotted using the Kaplan-Meier method, and statistical significance determined using the log-rank (Mantel-Cox) test. Group comparisons were made using the analysis of variance followed by the Newman-Keuls multiple-comparison test or unpaired t test. The other data were analyzed using one-way and two-way analyses of variance with GraphPad Prism 8.0. P < .05 was considered statistically significant.
More details of materials and methods are provided in the supplemental Data, available on the Blood website.
Results
SR604 antibody has significantly improved affinity toward APC, compared with HAPC1573
Firstly, we generated a humanized IgG1 chimeric antibody variant, Humab-14 of HAPC1573 and then performed systemic mutagenesis of every amino acid in the CDR of Humab-14 to improve its affinity toward human APC, which led to a new variant SR604. We also replaced the human IgG1 with IgG4 to improve its pharmacological activities. SR604 had 4 mutations in the CDR and exhibited enhanced affinity toward APC (Figure 1A; supplemental Table 1).
SR604 significantly improved binding affinity toward APC. (A) The sequence alignment of variable regions of HAPC1573, Humab-14, and SR604. CDRs are annotated using the Kabat numbering scheme. (B) Biacore sensorgrams (top) showing the association and dissociation curves of Humab-14 (left) and SR604 (right) binding to APC at different concentrations. The calculated affinity parameters are listed in the table at the bottom. (C) Model of APC-Humab-14 Fab complex computation simulated via the Protein-Protein Docking Module of BioLuminate. (D) Model of APC-SR604 Fab complex. (E-F) Protein-protein interactions between Humab-14 Fab (E) or SR604 Fab (F) and APC were calculated using the Protein Interaction Analysis module from BioLuminate (Schrödinger). The ribbon and surface maps were illustrated using Pymol (Schrödinger). RU, relative unit.
SR604 significantly improved binding affinity toward APC. (A) The sequence alignment of variable regions of HAPC1573, Humab-14, and SR604. CDRs are annotated using the Kabat numbering scheme. (B) Biacore sensorgrams (top) showing the association and dissociation curves of Humab-14 (left) and SR604 (right) binding to APC at different concentrations. The calculated affinity parameters are listed in the table at the bottom. (C) Model of APC-Humab-14 Fab complex computation simulated via the Protein-Protein Docking Module of BioLuminate. (D) Model of APC-SR604 Fab complex. (E-F) Protein-protein interactions between Humab-14 Fab (E) or SR604 Fab (F) and APC were calculated using the Protein Interaction Analysis module from BioLuminate (Schrödinger). The ribbon and surface maps were illustrated using Pymol (Schrödinger). RU, relative unit.
Surface plasmon resonance analysis demonstrated that SR604 bound to human APC with a KD of 0.288 nM relative to 17 nM of the parent Humab-14, which represents a 60-fold increase in the binding affinity (Figure 1B). SR604 had a much slower dissociation rate (Figure 1B). Both SR604 and Humab-14 did not bind to human protein C. SR604 also had no detectable binding to any other human plasma proteins (supplemental Figure 1A).
Sequence alignment between SR604 and Humab-14 indicated that 2 mutations located in heavy chain CDRs 1 and 2 (HCDR1 and HCDR2) and 2 in the light chain CDR 2 (LCDR2) contribute to the affinity enhancement (supplemental Table 1). To characterize the structural properties of the affinity-enhanced SR604, we performed computational model simulations of SR604 Fab and Humab-14 Fab in complex with APC, respectively. The homology modeling of SR604 Fab and Humab-14 Fab was carried out based on the HAPC1573 Fab and APC complex crystal structure from PDB 6M3C25; these are the atomic coordinates and structure factors of APC and HAPC1573 Fab complex structure, deposited in the Protein Data Bank, because the variable region of HAPC1573 shared high identity to those of the 2 Fab regions (Figure 1A). Subsequently, the models of APC-Humab-14 Fab and APC-SR604 Fab were achieved via molecular docking with some restraints (Figure 1C-D). By observing the close-up views of 2 APC-Fab models, the 4 mutations were located within the interface of Fab and APC. Among them, S55K in HCDR2 and N57R in LCDR2 showed their side chains closer to the antigen and generated new interaction (Figure 1D-F). In the surface maps, paratopes of SR604 Fab and Humab-14 Fab interactions within 5 Å of APC were marked in orange, and those within 3.6 Å, colored in red (supplemental Figure 1B-C). Based on the maps, the changed color of S55K and N57R mutations indicates enhanced interactions between the epitopes and paratopes. Apart from S55K and N57R, N31F in HCDR1 and E59G in LCDR2 individually and collectively may, minimally, lead to an improvement in the binding affinity and stability of the APC-SR604 Fab complex (supplemental Table 2). Furthermore, surface complementarity analysis between APC-SR604 Fab and APC-Humab-14 Fab showed that the N57R and S55K mutations improved surface complementarity of the Fab toward APC. Furthermore, the N57R and S55K mutations shortened the intramolecular distance between Fab and APC (supplemental Table 3). These data support that amino acid substitutions of S55K and N57R on the Fab are the critical mutations responsible for enhanced binding toward APC.
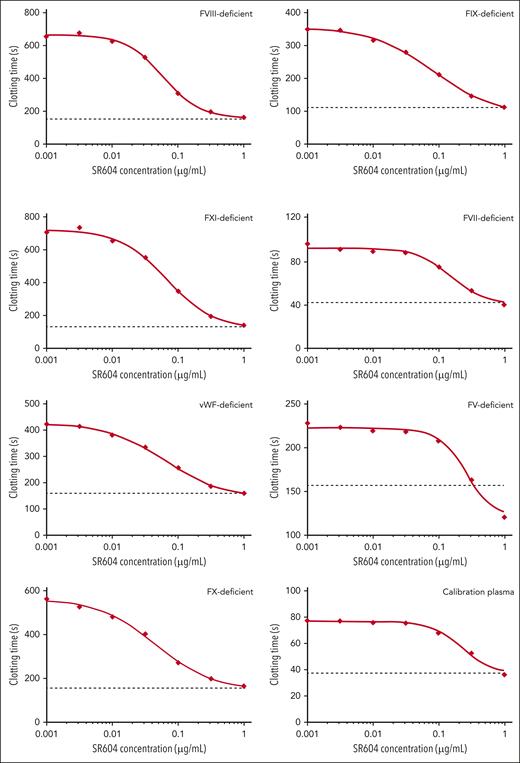
SR604 corrects prolonged clotting time of plasma deficient in different coagulation factors
We previously reported that HAPC1573 was able to reduce the clotting time of human plasma deficient in FV, FVII, FVIII, FIX, FX, FXI, or von Willebrand factor in the presence of protein C activation.5 However, HAPC1573 at a concentration of 50 μg/mL did not reach the total inhibitory effect on APC anticoagulant activity. Under similar conditions, we found that SR604 shortened the clotting time of human factor–deficient plasma at 90% inhibitory concentrations (IC90s) of 0.25 μg/mL for FVIII-deficient plasma and 0.49 μg/mL for FIX-deficient plasma (Figure 2), compared with the ∼50 μg/mL required for 90% inhibition by HAPC1573.5 These results suggest that the effective therapeutic concentrations of SR604 in patients with hemophilia could be as low as 0.25 μg/mL in plasma.
SR604 improves coagulation of human plasma deficient in coagulation factors. Clotting time of Protac-induced protein C activation in the activated partial thromboplastin time assay using plasma deficient in different clotting factors. The dotted lines in each assay represent the clotting time without Protac. The data are representative of duplicated assays. VWF, von Willebrand factor.
SR604 improves coagulation of human plasma deficient in coagulation factors. Clotting time of Protac-induced protein C activation in the activated partial thromboplastin time assay using plasma deficient in different clotting factors. The dotted lines in each assay represent the clotting time without Protac. The data are representative of duplicated assays. VWF, von Willebrand factor.
SR604 normalizes the hemostatic defects of humanized hemophilic mice
Unlike HAPC1573, which alone does not normalize bleeding time of humanized hemophilic mice, SR604 corrected the tail-bleeding time of mice expressing human APC with either hemophilia A or B (PROC+/+;F8−/− or PROC+/+;F9−/−) (Figure 3). We tested the hemostatic activity of different doses of SR604, that is, with 0.025, 0.05, 0.1, or 0.2 mg/kg body weight, measured based on the amount of bleeding after tail clipping in PROC+/+;F8−/− or PROC+/+;F9−/− mice. As expected, blood loss was significantly increased in the negative control group (PROC+/+;F8−/− or PROC+/+;F9−/− mice pretreated with phosphate-buffered saline) relative to wild-type (WT) mice. In contrast, treatment with human plasma–derived FVIII (1 IU per mouse; IV) 15 minutes before tail excision or treatment with human plasma–derived PCC (1 IU per mouse; IV), which were used as positive controls, significantly reduced bleeding in PROC+/+;F8−/− and PROC+/+;F9−/− mice. Notably, pretreatment with SR604, 24 hours before tail excision, exhibited a dose-dependent improvement of reduced bleeding in hemophilic mice, starting at a dose of 0.05 mg/kg. At a dose of 0.2 mg/kg, SR604 resulted in full correction to normal bleeding, similar to that of human plasma–derived FVIII or PCC (Figure 3).
SR604 normalizes the tail-bleeding time of humanized hemophilic mice. Tail-bleeding time was quantified as microliters of blood loss (hemoglobin content) per gram of mouse in 20 minutes after mouse tail transection. PROC+/+;F8−/− (A) and PROC+/+;F9−/−mice (B) were treated with PBS or SR604 antibody by subcutaneous injection at the indicated doses. After 24 hours, tail bleeding was measured for 20 minutes. As positive controls, a group of PROC+/+;F8−/− mice were injected with FVIII factor (1 IU per mouse) IV into the orbit for 15 minutes before the tail cutting, and a group of PROC+/+;F9−/−mice were injected with PCC (1 IU per mouse). Error bars represent standard error of the mean (n = 6 mice per group). ∗P < .05; ∗∗∗P < .001. PBS, phosphate-buffered saline.
SR604 normalizes the tail-bleeding time of humanized hemophilic mice. Tail-bleeding time was quantified as microliters of blood loss (hemoglobin content) per gram of mouse in 20 minutes after mouse tail transection. PROC+/+;F8−/− (A) and PROC+/+;F9−/−mice (B) were treated with PBS or SR604 antibody by subcutaneous injection at the indicated doses. After 24 hours, tail bleeding was measured for 20 minutes. As positive controls, a group of PROC+/+;F8−/− mice were injected with FVIII factor (1 IU per mouse) IV into the orbit for 15 minutes before the tail cutting, and a group of PROC+/+;F9−/−mice were injected with PCC (1 IU per mouse). Error bars represent standard error of the mean (n = 6 mice per group). ∗P < .05; ∗∗∗P < .001. PBS, phosphate-buffered saline.
SR604 significantly alleviated bleeding and inflammation of knee-joint injury in humanized hemophilic mice
Intra-articular bleeding is common in patients with hemophilia. To determine the efficacy of SR604 in controlling intra-articular bleeding, we performed the established knee-injury experiment, which mimics intra-articular bleeding, in PROC+/+;F8−/− and PROC+/+;F9−/− mice. There were 6 experimental groups, in addition to the WT groups: PROC+/+;F8−/− mice treated with or without 2 IU of FVIII, or PROC+/+;F9−/− mice treated with or without 2 IU of PCC as positive or negative controls, respectively. The remaining 2 groups were PROC+/+;F8−/− and PROC+/+;F9−/− mice treated with SR604 at 0.2 mg/kg body weight subcutaneously at 24 hours before knee injury, which was induced by needling the right knee of each mouse. All mice developed bleeding and swelling in the injured right knee, whereas the uninjured left knee was normal. The WT group did not die within 14 days. However, 3 of 6 mice in each factor-deficient group without pretreatment died within 6 days because of severe knee bleeding. In contrast, all PROC+/+;F8−/− and PROC+/+;F9−/− mice pretreated with SR604 antibody survived (Figure 4A).
SR604 significantly improves survival and ameliorates inflammation of knee-joint injury of humanized hemophilic mice. (A) Survival rates after injuries. (B) The change of the knee-joint diameter of mice calculated as percentage of the value before injury. Knee diameters were measured with an electronic caliper before needle injury and every other day after the injury; n = 6 mice per group. ∗∗P < .01. (C) Microscopic images of hematoxylin and eosin–stained knee joint tissues (original magnification ×20). Marked areas show inflammation and proliferation of the synovial and stromal linings. Bar graph represents quantification of the thickness of the inflamed and proliferated synovial and stromal linings; n = 3 mice per group. ∗P < .05; ∗∗∗P < .001.
SR604 significantly improves survival and ameliorates inflammation of knee-joint injury of humanized hemophilic mice. (A) Survival rates after injuries. (B) The change of the knee-joint diameter of mice calculated as percentage of the value before injury. Knee diameters were measured with an electronic caliper before needle injury and every other day after the injury; n = 6 mice per group. ∗∗P < .01. (C) Microscopic images of hematoxylin and eosin–stained knee joint tissues (original magnification ×20). Marked areas show inflammation and proliferation of the synovial and stromal linings. Bar graph represents quantification of the thickness of the inflamed and proliferated synovial and stromal linings; n = 3 mice per group. ∗P < .05; ∗∗∗P < .001.
The knee diameter of all experiment groups before the needle injury and every other day during the 2 weeks after the injury was measured with an electronic caliper, and the change of joint diameter was calculated as percentage of the preinjury value (Figure 4B). PROC+/+;F8−/− and PROC+/+;F9−/− mice reached the maximum knee swelling 2 days after injury, which was an ∼70% to 80% increase in diameter, whereas the joint diameter was increased by ∼20% in WT control mice, relative to that before the injury (Figure 4B). Pretreatment of PROC+/+;F8−/− and PROC+/+;F9−/− mice with SR604 reduced the knee swelling symptom in a degree comparable with that of pretreatment with FVIII or PCC (Figure 4B). Hematoxylin and eosin–stained sections of knees harvested 14 days after the needle-induced injury showed a greater expansion of the synovial and stromal linings with inflammation in PROC+/+;F8−/− and PROC+/+;F9−/− mice with injury relative to that of WT or PROC+/+;F8−/− or PROC+/+;F9−/− mice without injury. Pretreatment with SR640 considerably reduced the inflammation and proliferation of the synovial and stromal linings of PROC+/+;F8−/− or PROC+/+;F9−/− mice (Figure 4C).
SR604 does not interfere with the cytoprotection and endothelial barrier function of APC in hemophilic mice and exhibits an extended half-life in cynomolgus monkeys
We, and others, have shown that HAPC1573 could selectively block the anticoagulant activity of APC while maintaining APC noncoagulant activities, such as cytoprotection and endothelial barrier function, in vitro.5,25 To evaluate whether this critical therapeutic selectivity of the antibody changed after the antibody engineering, we tested SR604 in a LPS-induced systemic inflammatory model. Inhibition of the protein C pathway by HPC4, an antibody binding to the protein C activation peptide region in a Ca2+-dependent way to block protein C activation by the thrombin-thrombomodulin complex,28 exacerbated responses to a sublethal dose of LPS, leading to lethality (Figure 5A). In contrast, SR604 did not exacerbate the sublethal LPS responses in PROC+/+;F8−/− or PROC+/+;F9−/− mice. Sublethal LPS injection caused systemic inflammation and organ dysfunction in PROC+/+;F8−/− and PROC+/+;F9−/− mice, shown by elevated plasma inflammatory cytokine interleukin-6 and organ injury blood biochemistry markers such as alanine aminotransferase, aspartate aminotransferase, and lactate dehydrogenase (Figure 5B,C). HPC4 aggravated these parameters, whereas SR604 did not change them. In addition, hematoxylin and eosin–stained images and immunostaining with a pan inflammatory cell marker (CD45) showed that SR604 but not HPC4 reduced LPS-induced inflammatory cell infiltration in the lung tissue sections of PROC+/+;F8−/− and PROC+/+;F9−/− mice (Figure 5D,E; supplemental Figure 2A). Furthermore, using an in vitro model of endothelial cell barrier permeability, SR604 maintained the protective effect of APC on thrombin-induced endothelial permeability (supplemental Figure 2B) and enhanced its histone cleaving activities (supplemental Figure 3), similar to the functions of HAPC1573.21,25 These results indicate that SR604 maintains the cytoprotection and endothelial barrier function of APC. As a control, SR604 corrected the prolonged clotting time in the presence of protein C activation in plasma in vitro, comparable with that of HPC4 (supplemental Figure 4).
SR604 does not interfere with the cytoprotection and endothelial barrier function of APC in hemophilic mice. Eight-week-old male PROC+/+;F8−/− and PROC+/+;F9−/− mice were injected IV with either SR604 (0.5 mg/kg) or HPC4 (0.5 mg/kg), followed by intraperitoneal injection of LPS (10 mg/kg). (A) Survival rates of different groups of mice. Mice were examined every 12 hours for 3 days, and the survival rates were recorded after LPS challenge. (B-C) Plasma levels of alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, and interleukin-6 (IL-6) of mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. (D-E) Representative confocal images of immunofluorescent staining of cryosections of the lungs. CD31 marks endothelial cells and CD45 labels inflammatory cells, with 4′,6-diamidino-2-phenylindole (DAPI) used for nuclear staining. CD45+ cells were quantified using ImageJ software.
SR604 does not interfere with the cytoprotection and endothelial barrier function of APC in hemophilic mice. Eight-week-old male PROC+/+;F8−/− and PROC+/+;F9−/− mice were injected IV with either SR604 (0.5 mg/kg) or HPC4 (0.5 mg/kg), followed by intraperitoneal injection of LPS (10 mg/kg). (A) Survival rates of different groups of mice. Mice were examined every 12 hours for 3 days, and the survival rates were recorded after LPS challenge. (B-C) Plasma levels of alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, and interleukin-6 (IL-6) of mice. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. (D-E) Representative confocal images of immunofluorescent staining of cryosections of the lungs. CD31 marks endothelial cells and CD45 labels inflammatory cells, with 4′,6-diamidino-2-phenylindole (DAPI) used for nuclear staining. CD45+ cells were quantified using ImageJ software.
We also conducted a short-term toxicity study by subcutaneously injecting 100 mg/kg SR604 into PROC+/+;F8−/− or PROC+/+;F9−/− mice and found no toxicity effects in these mice after 14 days compared with mice injected with the same formulation of vehicle only (supplemental Figure 5).
Our pharmacokinetic study showed a half-life of 171 and 202 hours in cynomolgus monkeys when SR604 was dosed at 0.3 mg/kg intravenously or subcutaneously, respectively. In particular, the bioavailability of SR604 after subcutaneous injection was 106% (supplemental Figure 6).
Discussion
APC is a negative regulator in the coagulation cascade.17,18 APC is generated on demand locally when the vessel is injured and the endothelial surface converts protein C into APC to prevent local clotting.17 In patients with hemophilia, upon vessel injury, locally generated APC inhibits the hemostatic response. Inhibiting APC in hemophilia conditions should not have the risk of thrombotic side effects. We, and others, have demonstrated that the clotting time is not further shortened in the presence of protein C activation in vitro in clotting factor–deficient plasma, even with the maximum inhibition of APC activity.5,25,29
The APC concentration in circulation is ∼40 pM.26 This extremely low level of circulating APC provides an opportunity for a therapeutic mAb for prophylactic treatment with monthly or even bimonthly subcutaneous infusion. In this respect, we, and others, have reported that HAPC1573, which specifically blocks APC anticoagulant activity, significantly improves the bleeding defects in humanized protein C–knockin hemophilic mice and a monkey model of acquired hemophilia.5,25 However, because of its low affinity (∼10 nM KD) toward APC, HAPC1573 alone could not fully correct the tail-bleeding time in hemophilic mice.5 A dose as high as 30 mg/kg was needed to be fully effective in the monkey model of acquired hemophilia.25 To improve the affinity of the antibody, we screened all the amino acids in the CDRs of HAPC1573 and engineered the humanized SR604 with a combination of 4 amino acid mutations, which improves the affinity of the antibody toward APC to ∼0.288 nM KD, a 60-fold increase compared with its parent antibody HAPC1573.
In silico structural analysis revealed that the mutations of S55K in HCDR2 and N57R in LCDR2 on the Fab of SR604 contribute primarily to the improved binding to APC. HAPC1573 antibody interacts with a FVa-binding exosite of APC that includes an autolysis loop,25 which could lead to its inhibition of the anticoagulant activity of APC.25 Thus, SR604 has enhanced APC binding compared with HAPC1573, which results in a stronger inhibition of APC anticoagulant activity. SR604 does not interact with the Gla-domain of APC, which is required for the cytoprotective function of APC.24
Because of the dramatically improved affinity, the IC90 for SR604 is as low as 0.25 μg/mL compared with 50 μg/mL for HAPC1573 in the antibody functional inhibitory assay for APC anticoagulant activity in factor-deficient plasma in vitro Protac-APTT experiments. Interestingly, SR604, at a dose of 0.05 mg/kg subcutaneous injection, showed a nearly complete protective effect in the tail-bleeding experiments in mice with either hemophilia A or B, consistent with the in vitro IC90 data. At a higher dose of 0.2 mg/kg, subcutaneous injections of SR604 exhibited a similar hemostatic effect as FVIII or PCC treatments (1 IU per mouse) in the tail-bleeding experiments. Together with our SR604 pharmacokinetic results in monkeys, we anticipate that a low-dose regimen of SR604 subcutaneous injection biweekly or monthly (which may not only improve patient adherence but also prevent injection-related adverse reactions) would be effective in future human clinical trials for prophylactic treatment of patients with hemophilia A or B with or without inhibitors.
Prophylactic treatment of patients with hemophilia is a lifelong therapeutic process. Infection and inflammation might occur in these patients unavoidably. Inhibition of the protein C pathway by HPC4 antibody converts a sublethal Escherichia coli challenge into a lethal response in baboons.22,30 HPC4 antibody inhibits protein C activation and therefore eliminates proteolytic activities of APC for anticoagulation as well as cytoprotection and anti-inflammation.28 In this study, we recapitulated the exacerbated effects of HPC4 in PROC+/+;F8−/− and PROC+/+;F9−/− mice with a sublethal dose of LPS challenge. Inhibition of protein C anticoagulant activity in hemophilic mice should have beneficial effects in rebalancing coagulation and anticoagulation; however, the lethal effect of HPC4 in hemophilic mice, challenged with a sublethal dose of LPS, highlights the critical noncoagulant functions mediated by APC in vivo. Our data show, to our knowledge, for the first time, that cytoprotective and/or anti-inflammatory effects of APC independent of anticoagulation could play a pivotal role in inflammatory diseases without thrombotic complications. Therefore, we expect that long-term application of SR604, which maintains the cytoprotective and endothelial barrier function of APC, would not increase the susceptibility of patients with hemophilia to inflammatory disease.
One major advantage of antibody-based therapeutics, such as hemlibra or marstacimab, for hemophilia treatment is that subcutaneous injections allow longer intervals compared with standard IV injections of coagulant products within a week.31 However, transiently high concentrations of nonfactor coagulant or coagulant-facilitated products at local subcutaneous injection sites might trigger unwanted local coagulation and induce thrombotic side effects around injection sites. Based on the mode of action of SR604, which specifically inhibits APC anticoagulant activity, in the conditions of patients with hemophilia who are deficient in coagulant factors, high concentrations of SR604 in the subcutaneous injection sites would not cause unwanted thrombotic side effects. We verified this hypothesis by injecting 100 mg/kg SR604 subcutaneously into PROC+/+;F8−/− and PROC+/+;F9−/− mice, a dose that is 1000 times greater than our planned dosage used in human clinical trials, and we observed no acute toxic side effects.
Patients with hemophilia often develop inhibitors, which precludes effective treatment with factors.7,8 In contrast, SR604 bypasses the introduction of factors and should be effective in patients with hemophilia with inhibitors. Studies in nonhuman primates have shown that the parent antibody HAPC1573 can prevent bleeding in animals sensitized with an anti-FVIII antibody.25 We anticipate that SR604 could be used for routine prophylaxis to prevent or reduce the bleeding episodes in patients deficient in coagulation factors with or without inhibitors.
In conclusion, our in vitro and in vivo data indicate that SR604, an affinity-enhanced humanized APC antibody, is a novel therapeutic agent that may have advantages over factors or nonfactor products on the market and in development for patients with congenital factor deficiencies.
Acknowledgments
The authors thank JOINN Laboratories for their pharmacokinetic studies in cynomolgus monkeys and ChemPartner Biologicals (Shanghai) for manufacturing SR604.
This work was supported by funds from the Jiangsu Provincial Key Medical Center (YXZXA2016002) (C.R. and D.W.), the Priority Academic Program Development of Jiangsu Higher Education Institutions (C.R. and D.W.), Anhui Province Excellent Youth Program in Universities (2022AH030121) (Y.J.), Foundation of Key Research and Development Program of Anhui Province (202104j07020020) (Y.J.), and the National Natural Science Foundation of China (82200146, 82230005, 82020108003, 82070143, and 81730003) (D.W., Y.H., and Y.J.).
Authorship
Contribution: J.X., M.J., Y.H., D.W., and L.X. designed and supervised research; F.Y., J.H., J.Y., B.Z., L.H., Z.M., L.J.C., Z.X., C.J., T.L., and T.T.C.Y. performed research; J.X., M.J., F.Y., Y.J., L.C., X.B., C.R., N.L.E., C.T.E., Y.H., D.W., and L.X. analyzed data and contributed to the manuscript preparation; and J.X., Y.H., D.W., Y.J., and L.X. wrote the maniscript.
Conflict-of-interest disclosure: L.C., J.Y., Z.X., and J.X. are employees of Shanghai RAAS Blood Products Co, Ltd. L.C., J.Y, Z.X, J.X., and C.T.E. are listed as inventors on patents and patent applications related to HAPC1573, SR604, and humanized protein C mouse models. The remaining authors declare no competing financial interests.
Correspondence: Jun Xu, Shanghai RAAS Blood Products Co, Ltd, 2009 Wangyuan Road, Shanghai 201401, China; e-mail: junxu@raas-corp.com; Depei Wu, Jiangsu Institute of Hematology, 188 Shizi St, Suzhou, Jiangsu 215006, China; e-mail: wudepei@suda.edu.cn; and Yue Han, Jiangsu Institute of Hematology, 188 Shizi St, Suzhou, Jiangsu 215006, China; e-mail: hanyue@suda.edu.cn.
References
Author notes
∗M.J., F.Y., Y.J., and L. Cheng are joint first authors.
Data are available on request to the corresponding author, Jun Xu (junxu@raas-corp.com).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal