Key Points
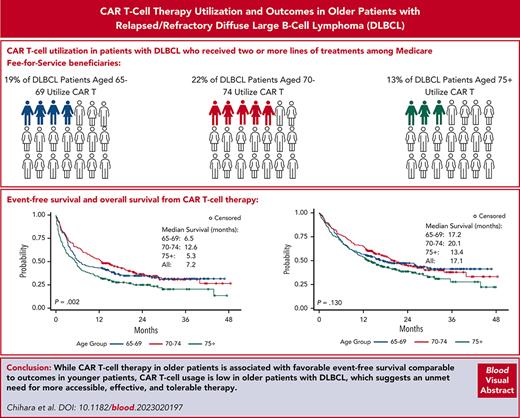
CAR T-cell therapy is associated with favorable event-free survival in older patients, comparable to outcomes in younger patients.
CAR T-cell therapy use is low in older patients, demonstrating an unmet need for more accessible, effective, and tolerable therapy.
Abstract
The emergence of chimeric antigen receptor (CAR) T-cell therapy has changed the treatment landscape for diffuse large B-cell lymphoma (DLBCL); however, real-world experience reporting outcomes among older patients treated with CAR T-cell therapy is limited. We leveraged the 100% Medicare fee-for-service claims database and analyzed outcomes and cost associated with CAR T-cell therapy in 551 older patients (aged ≥65 years) with DLBCL who received CAR T-cell therapy between 2018 and 2020. CAR T-cell therapy was used in third line and beyond in 19% of patients aged 65 to 69 years and 22% among those aged 70 to 74 years, compared with 13% of patients aged ≥75 years. Most patients received CAR T-cell therapy in an inpatient setting (83%), with an average length of stay of 21 days. The median event-free survival (EFS) following CAR T-cell therapy was 7.2 months. Patients aged ≥75 years had significantly shorter EFS compared with patients aged 65 to 69 and 70 to 74 years, with 12-month EFS estimates of 34%, 43%, and 52%, respectively (P = .002). The median overall survival was 17.1 months, and there was no significant difference by age groups. The median total health care cost during the 90-day follow-up was $352 572 and was similar across all age groups. CAR T-cell therapy was associated with favorable effectiveness, but the CAR T-cell therapy use in older patients was low, especially in patients aged ≥75 years, and this age group had a lower rate of EFS, which illustrates the unmet need for more accessible, effective, and tolerable therapy in older patients, especially those aged ≥75 years.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1104.
Disclosures
CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declares no competing financial interests.
Learning Objectives
Upon completion of this activity, participants will:
Assess clinical characteristics and use of chimeric antigen receptor (CAR) T-cell therapy among older patients with diffuse large B-cell lymphoma (DLCBL), based on an analysis of the 100% Medicare Fee-for-Service claims database between 2018 and 2020
Evaluate outcomes and costs among older patients with DLCBL treated with CAR T-cell therapy, based on an analysis of the 100% Medicare Fee-for-Service claims database between 2018 and 2020
Determine the clinical and public health implications of outcomes, use, and costs among older patients with DLCBL treated with CAR T-cell therapy, based on an analysis of the 100% Medicare Fee-for-Service claims database between 2018 and 2020
Release date: September 21, 2023; Expiration date: September 21, 2024
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoma, with annual estimated new cases of ≈25 000 in the United States.1 The median age of onset of DLBCL is 66 years, and close to 30% of patients are diagnosed at age ≥75 years.2 The percentage of the population aged ≥65 years is projected to increase from 15% in 2016 to 21% in 2030, according to the US census, and thus the prevalence of older patients with DLBCL is expected to grow with the aging population.3 Treatment of this geriatric patient population can be challenging because of multiple factors, such as comorbidities, poor performance status (PS), and potentially higher-risk DLBCL biology.4
The treatment landscape for relapsed/refractory DLBCL has dramatically evolved in recent years. Historically, platinum-based chemotherapy, followed by high-dose therapy/autologous stem cell transplant (auto-SCT), was the only option for long-term remission for relapsed/refractory DLBCL5,6; however, older patients are frequently considered ineligible for this treatment, except fit patients,7 and survival outcomes for those who did not receive auto-SCT following recurrence were dismal.8 Development of CD19-directed autologous chimeric antigen receptor (CAR) T-cell therapy has resulted in a paradigm shift in the treatment of relapsed/refractory DLBCL,9-11 providing an effective treatment option for older patients who are not necessarily candidates for auto-SCT. CAR T-cell therapy can achieve long-term remissions for a portion of patients experiencing a recurrence of DLBCL often independent of characteristics, such as age.9,11-14 The US Food and Drug Administration first approved axicabtagene ciloleucel (axi-cel) in late 2017, soon followed by tisagenlecleucel in 2018, and then lisocabtagene maraleucel in 2021 for patients with DLBCL or high-grade B-cell lymphomas who are relapsed or refractory to ≥2 prior lines of therapy based on single-arm phase 2 trials.9,11,13
Since the approval, multiple studies have reported on the real-world experience (RWE) of CAR T-cell therapy, confirming the effectiveness of this treatment, depending on various risk factors, such as serum lactate dehydrogenase, PS, and tumor burden before CAR T-cell infusion.15-22 The outcomes of CAR T-cell therapy in older patients also have been evaluated in both clinical trials and RWE studies.23-25 In the ZUMA-1 study, 27 patients aged ≥65 years and treated with axi-cel showed a 92% overall response rate and 42% of patients achieved ongoing response with a minimum of 24 months of follow-up at the time of the report.24 Jacobson et al analyzed 1297 patients who received commercial axi-cel and showed that response rate was higher among patients aged ≥65 years.16 Ram et al summarized the outcomes of 41 patients with DLBCL aged ≥70 years who received CAR T-cell therapy, most of them with tisagenlecleucel (81%), and reported that there was no significant difference in the rate of cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), overall response rate, and progression-free survival in older patients compared with younger patients.23 However, studies had limited data, particularly among patients aged >70 years, evaluating how age or other factors affect survival outcomes among older patients who received CAR T-cell therapy. Moreover, no study has described health care use and cost associated with CAR T-cell therapy in older patients. Therefore, we evaluated the clinical outcomes and economic impact associated with CAR T-cell therapy in older patients with DLBCL by leveraging a Medicare claims database.
Materials and methods
Data source and patient selection
This study used the Centers for Medicare & Medicaid Services–sourced 100% Medicare fee-for-service (FFS) Parts A/B/D claims data, which cover all patients who are treated under Medicare FFS plans, spanning from 1 October 2015 through 31 December 2020. Data included all Part A/B medical encounters for Medicare FFS beneficiaries, including hospital claims, emergency department (ED) visits, skilled nursing facility stays, hospital outpatient services/ambulatory surgical center services, clinic visits, home health services/durable medical equipment, and hospice care. Part D data include all retail and mail-order pharmacy prescriptions, whereas the master beneficiary summary file includes patient demographics, enrollment dates, and, where applicable, date of death. Informed consent for the study was waived because of the retrospective nature of the study using registry claim data.
Patients included in the study had at least 1 inpatient or 2 outpatient claims with an International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM), code of DLBCL between 1 April 2016 and 1 December 2020, followed by a claim with an International Classification of Diseases, Tenth Revision (ICD-10), procedure code, Healthcare Common Procedure Coding System code, National Drug Code, or Diagnosis-Related Group code for CAR T-cell therapy, which must have been observed between 1 January 2018 and 31 December 2020. All patients were required to be aged ≥65 years on the date of CAR T-cell administration, and patients who received CAR T-cell therapy on a clinical trial were excluded. The original data are available from the Medicare FFS claims database.
Statistical analysis
Demographic characteristics, including age, sex, and an urbanicity residence indicator, were assessed on the date of CAR T-cell administration. The Charlson Comorbidity Index (CCI),26 excluding the diagnosis of DLBCL, was calculated during the 6-month period preceding the CAR T-cell administration for patients with at least 6 months of continuous enrollment before CAR T-cell administration. To accurately capture all lymphoma treatments, the line of treatment was calculated only in patients who have continuous enrollment in Medicare for at least 2 years before their first DLBCL diagnosis in the data. Bridging therapy was defined as any treatment within 28 days before CAR T-cell administration, and classified as corticosteroids, radiotherapy, and chemotherapy/targeted therapies. Because claims data do not capture progression of lymphoma, event-free survival (EFS) was used as a proxy of treatment success in this study; and an event was defined as initiation of next lymphoma treatment or death from any cause. For those patients with at least 90 days of follow-up, health care resource use (HCRU) and total health care cost over the 90-day period following CAR T-cell administration were measured. HCRU included inpatient, outpatient, and ED service use. Total health care costs, including intensive care unit cost, were calculated and adjusted for inflation using the medical care component of the Consumer Price Index from the US Bureau of Labor Statistics and standardized to 2020 US dollars.27
Descriptive analyses included the presentation of means, medians, and standard deviations (SDs) for continuous variables, and frequencies and proportions for categorical variables. Analyses were stratified by age groups (65-69 vs 70-74 vs ≥75) and CAR T-cell administration place of service (inpatient vs outpatient). EFS and overall survival (OS) were calculated using Kaplan-Meier analyses from the date of CAR T-cell administration. Differences in EFS and OS across age groups was assessed using the log-rank test, with the significance level set at .05. Univariate and multivariate Cox proportional hazards models were performed to analyze the association between patient characteristics (age, sex, urbanicity, bridging therapy, and CCI) and EFS or OS. All analyses were conducted with SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
From an initial sample of 78 839 patients with a diagnosis of DLBCL between 1 April 2016 and 1 December 2020, 70 265 patients were aged ≥65 years, and a total of 551 patients (aged 65-69 years, n = 202; aged 70-74 years, n = 176; and aged ≥75 years, n = 173) met all inclusion and exclusion criteria (supplemental Figure 1, available on the Blood website). Within the patients who had at least 2 years of continuous enrollment before their first DLBCL diagnosis (n=29 452), for whom line of therapy was delineated, ≈19% of patients aged 65 to 69 years 22% of patients aged 70 to 74 years received CAR T-cell therapy compared with only 13% of patients aged ≥75 years who received CAR T-cell therapy during the course of treatment after ≥2 lines of treatment.
The median age of all the patients was 72 years (range, 65-90 years), 54% of patients were men, and 81% of patients were living in an urban/suburban area (Table 1). Among patients (n = 516) who had at least 6 months of continuous enrollment before CAR T-cell administration, the median CCI score was 4, with 41% of patients presenting a score of ≥5, and there was no significant difference among age groups. Most patients received CAR T-cell therapy in an inpatient setting (n = 456; 83%), with a mean hospital length of stay of 21.4 days (SD, ±16.2 days). A total of 262 patients (48%) received bridging therapy, with the most common treatments being chemotherapy or targeted therapy (29%), corticosteroids (28%), and radiotherapy (11%).
Patient characteristics
| Characteristic . | Patients aged 65-69 y . | Patients aged 70-74 y . | Patients aged ≥75 y . | All patients . | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 202) . | (n = 176) . | (n = 173) . | (n = 551) . | |||||
| Age, median (range), y | 67 | (65-69) | 72 | (70-74) | 78.6 | (75-90) | 72.2 | (65-90) |
| Urban/suburban, n (%) | 160 | (79.2) | 142 | (80.7) | 142 | (82.1) | 444 | (80.5) |
| Male sex, n (%) | 108 | (53.5) | 93 | (52.8) | 98 | (56.6) | 299 | (54.3) |
| Baseline Charlson Comorbidity Index, mean (SD)∗ | 5 | (3.2) | 5 | (3.3) | 5 | (3.3) | 5 | (3.24) |
| Median (range) | 4 | (0-15) | 4 | (0-15) | 4 | (0-15) | 4 | (0-15) |
| Bridging therapies, n (%)† | ||||||||
| Any therapy | 102 | (50.5) | 69 | (39.2) | 91 | (52.6) | 262 | (47.5) |
| Chemotherapy or targeted therapy | 64 | (31.7) | 41 | (23.3) | 55 | (31.8) | 160 | (29.0) |
| Corticosteroids | <50 | (∼) | <50 | (∼) | 23 | (13.3) | 73 | (27.9) |
| Radiotherapy | <11 | (∼)‡ | <11 | (∼)‡ | 13 | (7.5) | 29 | (11.1) |
| CAR T-cell administration setting, n (%) | ||||||||
| Inpatient | 171 | (84.6) | 155 | (88.1) | 130 | (75.1) | 456 | (82.8) |
| Length of stay, mean (SD), d | 19.7 | (12.36) | 24.2 | (21.15) | 20.5 | (13.09) | 21.4 | (16.2) |
| Outpatient | 31 | (15.3) | 21 | (11.9) | 43 | (24.9) | 95 | (17.2) |
| Characteristic . | Patients aged 65-69 y . | Patients aged 70-74 y . | Patients aged ≥75 y . | All patients . | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 202) . | (n = 176) . | (n = 173) . | (n = 551) . | |||||
| Age, median (range), y | 67 | (65-69) | 72 | (70-74) | 78.6 | (75-90) | 72.2 | (65-90) |
| Urban/suburban, n (%) | 160 | (79.2) | 142 | (80.7) | 142 | (82.1) | 444 | (80.5) |
| Male sex, n (%) | 108 | (53.5) | 93 | (52.8) | 98 | (56.6) | 299 | (54.3) |
| Baseline Charlson Comorbidity Index, mean (SD)∗ | 5 | (3.2) | 5 | (3.3) | 5 | (3.3) | 5 | (3.24) |
| Median (range) | 4 | (0-15) | 4 | (0-15) | 4 | (0-15) | 4 | (0-15) |
| Bridging therapies, n (%)† | ||||||||
| Any therapy | 102 | (50.5) | 69 | (39.2) | 91 | (52.6) | 262 | (47.5) |
| Chemotherapy or targeted therapy | 64 | (31.7) | 41 | (23.3) | 55 | (31.8) | 160 | (29.0) |
| Corticosteroids | <50 | (∼) | <50 | (∼) | 23 | (13.3) | 73 | (27.9) |
| Radiotherapy | <11 | (∼)‡ | <11 | (∼)‡ | 13 | (7.5) | 29 | (11.1) |
| CAR T-cell administration setting, n (%) | ||||||||
| Inpatient | 171 | (84.6) | 155 | (88.1) | 130 | (75.1) | 456 | (82.8) |
| Length of stay, mean (SD), d | 19.7 | (12.36) | 24.2 | (21.15) | 20.5 | (13.09) | 21.4 | (16.2) |
| Outpatient | 31 | (15.3) | 21 | (11.9) | 43 | (24.9) | 95 | (17.2) |
∼, value withheld to comply with the CMS policy of minimizing the risk to identify patients.
Total 516 patients are included in baseline Charlson Comorbidity Index calculation.
Presence of therapy in the 28-day period before CAR T-cell treatment, excluding therapies administered on the same day as CAR T-cell therapy. Cyclophosphamide and fludarabine administered in the period 10 days before CAR T-cell therapy are also excluded.
The Centers for Medicare & Medicaid Services (CMS) cell size suppression policy sets minimum thresholds for the display of CMS data, in which no cell (eg, admissions, discharges, patients, and services) containing a value of 1 to 10 can be reported directly.
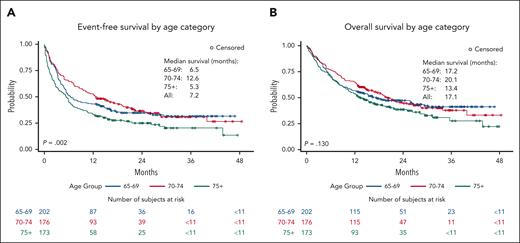
With a median follow-up of 11.9 months (range, 0.03-45.3 months) after the CAR T-cell administration, 186 patients (34%) received a next treatment for DLBCL and 316 patients (57%) died. The median EFS following CAR-T-cell therapy in all patients was 7.2 months (95% confidence interval [CI], 6.0-9.7 months). Patients aged ≥75 years showed significantly shorter EFS (median, 5.3 months; 95% CI, 3.4-6.6 months) compared with patients aged 65 to 69 years (median, 6.5 months; 95% CI, 5.1-11.6 months) and aged 70 to 74 years (median, 12.6 months; 95% CI, 9.2-18.8 month); and the 12-month EFS rate was 43% (95% CI, 36%-50%), 52% (95% CI, 45%-60%), and 34% (95% CI, 27%-41%) in those aged 65 to 69, 70 to 74, and ≥75 years, respectively (Figure 1A: P = .002). The 24-month EFS was 35%, 37%, and 25% in those aged 65 to 69, 70 to 74, and ≥75 years, respectively. The median OS was 17.1 months (95% CI, 14.2-21.0 months) in all patients. The 12-month OS estimate was 57% (95% CI, 50%-63%), 64% (95% CI, 58%-72%), and 54% (95% CI, 46%-61%) in those aged 65 to 69, 70 to 74, and ≥75 years, respectively, and there was no significant difference in OS by age groups (Figure 1B: P = .130).
Kaplan-Meier curve by age category. Event-free survival (A) and overall survival (B) after CAR T-cell therapy infusion by age category.
Kaplan-Meier curve by age category. Event-free survival (A) and overall survival (B) after CAR T-cell therapy infusion by age category.
Age ≥75 years (vs 65-69 and vs 70-74 years), use of bridging therapy, and baseline CCI ≥5 were significantly associated with shorter EFS by univariate analysis (Table 2). Patients with CCI ≥5 has associated inferior EFS compared with those with CCI <5 in each age group (1-year EFS, age 65-69 years: 52% vs 36%; age 70-74 years: 54% vs 48%; age ≥75 years: 41% vs 24%). Multivariate analysis demonstrated that CCI ≥5 was independently associated with EFS to age and use of bridging therapy (hazard ratio, 1.56; 95% CI, 1.26-1.92). Use of bridging therapy and baseline CCI ≥5 were associated with inferior OS by univariate analysis (Table 2), and multivariate analysis demonstrated that CCI ≥5 was independently associated with OS to age and use of bridging therapy (hazard ratio, 1.58; 95% CI, 1.26-1.99).
Cox proportional hazards model for EFS and OS
| Characteristic . | Categories . | EFS . | OS . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate . | Multivariate . | Univariate . | Multivariate . | ||||||||||
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | ||
| Age groups | ≥75 vs 65-69 y | 1.37 | 1.07-1.74 | .011 | 1.41 | 1.10-1.82 | .007 | 1.25 | 0.96-1.62 | .105 | 1.2 | 0.91-1.58 | .188 |
| ≥75 vs 70-74 y | 1.54 | 1.19-1.98 | .001 | 1.46 | 1.13-1.89 | .004 | 1.29 | 0.98-1.70 | .066 | 1.2 | 0.90-1.58 | .207 | |
| Sex | Male vs female | 0.99 | 0.81-1.22 | .943 | 0.92 | 0.75-1.14 | .449 | 1.06 | 0.85-1.33 | .577 | 1 | 0.80-1.26 | .973 |
| Urban/suburban residence | Rural vs urban | 1.14 | 0.88-1.47 | .317 | — | — | 1.22 | 0.93-1.60 | .158 | — | — | ||
| Bridging therapy | Present vs absent | 1.34 | 1.09-1.64 | .005 | 1.27 | 1.03-1.56 | .028 | 1.49 | 1.19-1.86 | <.001 | 1.39 | 1.11-1.75 | .005 |
| Charlson Comorbidity Index | ≥5 vs 0-4 | 1.57 | 1.28-1.94 | <.0001 | 1.56 | 1.26–1.92 | <.0001 | 1.63 | 1.30-2.05 | <.0001 | 1.58 | 1.26-1.99 | <.0001 |
| Characteristic . | Categories . | EFS . | OS . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate . | Multivariate . | Univariate . | Multivariate . | ||||||||||
| HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | HR . | 95% CI . | P value . | ||
| Age groups | ≥75 vs 65-69 y | 1.37 | 1.07-1.74 | .011 | 1.41 | 1.10-1.82 | .007 | 1.25 | 0.96-1.62 | .105 | 1.2 | 0.91-1.58 | .188 |
| ≥75 vs 70-74 y | 1.54 | 1.19-1.98 | .001 | 1.46 | 1.13-1.89 | .004 | 1.29 | 0.98-1.70 | .066 | 1.2 | 0.90-1.58 | .207 | |
| Sex | Male vs female | 0.99 | 0.81-1.22 | .943 | 0.92 | 0.75-1.14 | .449 | 1.06 | 0.85-1.33 | .577 | 1 | 0.80-1.26 | .973 |
| Urban/suburban residence | Rural vs urban | 1.14 | 0.88-1.47 | .317 | — | — | 1.22 | 0.93-1.60 | .158 | — | — | ||
| Bridging therapy | Present vs absent | 1.34 | 1.09-1.64 | .005 | 1.27 | 1.03-1.56 | .028 | 1.49 | 1.19-1.86 | <.001 | 1.39 | 1.11-1.75 | .005 |
| Charlson Comorbidity Index | ≥5 vs 0-4 | 1.57 | 1.28-1.94 | <.0001 | 1.56 | 1.26–1.92 | <.0001 | 1.63 | 1.30-2.05 | <.0001 | 1.58 | 1.26-1.99 | <.0001 |
—, this variable was not included in the multivariate analysis since it was not prognostic by univariate analysis. HR, hazard ratio.
A total of 445 patients (81%) were included in the HCRU and costs analyses (Table 3). Among patients who received CAR T-cell therapy in an inpatient setting, 29% had at least 1 rehospitalization after discharge, and 30% had at least 1 ED visit during the 90 days following CAR T-cell administration. The readmission rate was 34%, 22%, and 29% in those aged 65 to 69, 70 to 74, and ≥75 years, respectively (P = .110). The mean outpatient visits for those patients who received CAR T-cell therapy in an inpatient setting were 17 (SD, ±8.7) in a 90-day period. Among patients who received CAR T-cell therapy in an outpatient setting, 42% experienced at least 1 hospitalization during the 90-day period following CAR T-cell administration, with patients aged ≥75 years showing lower rate of hospitalization (65-69 years: 54%; 70-74 years: 50%; and ≥75 years: 30%; P = .006), and 32% had at least 1 ED visit. The mean outpatient visits in the 90-day period following CAR T-cell therapy were 19 (SD, ±9.4). Overall, the median total health care cost during the 90-day follow-up period (inclusive of the CAR T-cell administration) in all patients was $352 572. The mean cost was $311 699, $296 192, and $271 767 in age groups of 65 to 69, 70 to 74, and ≥75 years, respectively (P = .199).
Health care resource use and costs by age category
| Variable . | Aged 65-69 y . | Aged 70-74 y . | Aged ≥75 y . | Total . | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 168) . | (n = 143) . | (n = 134) . | (n = 445) . | |||||
| Total health care costs, $ | ||||||||
| Mean (SD)∗ | 311 699 | (189 161) | 296 192 | (196 115) | 271 767 | (190 975) | 294 692 | (192 232) |
| Median | 364 036 | 342 099 | 333 698 | 352 572 | ||||
| Service use | ||||||||
| Among patients receiving CAR T-cell therapy in an inpatient setting, n (%) | 144 | (85.7) | 125 | (87.4) | 97 | (72.4) | 366 | (82.2) |
| Rehospitalization† | ||||||||
| ≥1 visit, n (%) | 49 | (34.0) | 28 | (22.4) | 28 | (28.9) | 105 | (28.7) |
| Total visits, mean (SD) | 1.3 | (0.7) | 1.7 | (1) | 1.5 | (0.9) | 1.5 | (0.8) |
| Length of stay, mean (SD), d | 8.22 | (6.97) | 6.62 | (5.08) | 7.83 | (7.5) | 7.62 | (6.59) |
| ED services‡ | ||||||||
| ≥1 visit, n (%) | 47 | (32.6) | 37 | (29.6) | 27 | (27.8) | 111 | (30.3) |
| Total visits, mean (SD) | 1.6 | (0.9) | 1.7 | (0.9) | 1.7 | (0.9) | 1.6 | (0.9) |
| Outpatient services | ||||||||
| ≥1 visit, n (%) | 144 | (100.0) | 125 | (100.0) | 97 | (100.0) | 366 | (100.0) |
| Total visits, mean (SD) | 17.7 | (8.9) | 18 | (8.7) | 16.3 | (8.6) | 17.4 | (8.7) |
| Among patients receiving CAR T-cell therapy in an outpatient setting, n (%) | 24 | (14.3) | 18 | (12.6) | 37 | (27.6) | 79 | (17.8) |
| Follow-up inpatient services† | ||||||||
| ≥1 visit, n (%) | 13 | (54.2) | <11 | (∼)§ | 11 | (29.70) | 33 | (41.80) |
| Total visits, mean (SD) | 1.4 | (0.7) | 1.3 | (0.7) | 1.4 | (0.5) | 1.4 | (0.6) |
| Length of stay, mean (SD), d | 6.28 | (4.47) | 7.75 | (7) | 8.87 | (7.63) | 7.53 | (6.31) |
| ED services‡ | ||||||||
| ≥1 visit, n (%) | <11 | (∼)§ | <11 | (∼)§ | 11 | (29.70) | 25 | (31.60) |
| Total visits, mean (SD) | 1.8 | (0.9) | 2.3 | (1.9) | 1.9 | (0.9) | 1.9 | (1.1) |
| Outpatient services (not inclusive of initial CAR T-cell therapy visit) | ||||||||
| ≥1 visit, n (%) | 24 | (100.0) | 18 | (100.0) | 37 | (100.0) | 79 | (100.0) |
| Total visits, mean (SD) | 21.8 | (10.5) | 17.9 | (9.9) | 17.6 | (8.2) | 19 | (9.4) |
| Variable . | Aged 65-69 y . | Aged 70-74 y . | Aged ≥75 y . | Total . | ||||
|---|---|---|---|---|---|---|---|---|
| (n = 168) . | (n = 143) . | (n = 134) . | (n = 445) . | |||||
| Total health care costs, $ | ||||||||
| Mean (SD)∗ | 311 699 | (189 161) | 296 192 | (196 115) | 271 767 | (190 975) | 294 692 | (192 232) |
| Median | 364 036 | 342 099 | 333 698 | 352 572 | ||||
| Service use | ||||||||
| Among patients receiving CAR T-cell therapy in an inpatient setting, n (%) | 144 | (85.7) | 125 | (87.4) | 97 | (72.4) | 366 | (82.2) |
| Rehospitalization† | ||||||||
| ≥1 visit, n (%) | 49 | (34.0) | 28 | (22.4) | 28 | (28.9) | 105 | (28.7) |
| Total visits, mean (SD) | 1.3 | (0.7) | 1.7 | (1) | 1.5 | (0.9) | 1.5 | (0.8) |
| Length of stay, mean (SD), d | 8.22 | (6.97) | 6.62 | (5.08) | 7.83 | (7.5) | 7.62 | (6.59) |
| ED services‡ | ||||||||
| ≥1 visit, n (%) | 47 | (32.6) | 37 | (29.6) | 27 | (27.8) | 111 | (30.3) |
| Total visits, mean (SD) | 1.6 | (0.9) | 1.7 | (0.9) | 1.7 | (0.9) | 1.6 | (0.9) |
| Outpatient services | ||||||||
| ≥1 visit, n (%) | 144 | (100.0) | 125 | (100.0) | 97 | (100.0) | 366 | (100.0) |
| Total visits, mean (SD) | 17.7 | (8.9) | 18 | (8.7) | 16.3 | (8.6) | 17.4 | (8.7) |
| Among patients receiving CAR T-cell therapy in an outpatient setting, n (%) | 24 | (14.3) | 18 | (12.6) | 37 | (27.6) | 79 | (17.8) |
| Follow-up inpatient services† | ||||||||
| ≥1 visit, n (%) | 13 | (54.2) | <11 | (∼)§ | 11 | (29.70) | 33 | (41.80) |
| Total visits, mean (SD) | 1.4 | (0.7) | 1.3 | (0.7) | 1.4 | (0.5) | 1.4 | (0.6) |
| Length of stay, mean (SD), d | 6.28 | (4.47) | 7.75 | (7) | 8.87 | (7.63) | 7.53 | (6.31) |
| ED services‡ | ||||||||
| ≥1 visit, n (%) | <11 | (∼)§ | <11 | (∼)§ | 11 | (29.70) | 25 | (31.60) |
| Total visits, mean (SD) | 1.8 | (0.9) | 2.3 | (1.9) | 1.9 | (0.9) | 1.9 | (1.1) |
| Outpatient services (not inclusive of initial CAR T-cell therapy visit) | ||||||||
| ≥1 visit, n (%) | 24 | (100.0) | 18 | (100.0) | 37 | (100.0) | 79 | (100.0) |
| Total visits, mean (SD) | 21.8 | (10.5) | 17.9 | (9.9) | 17.6 | (8.2) | 19 | (9.4) |
∼, value withheld to comply with the CMS policy of minimizing the risk to identify patients.
Total Medicare payments on all accepted inpatient, outpatient, skilled nursing facility, home health agency, hospice, professional, and pharmacy Medicare FFS claims found within 90 days after CAR T-cell therapy, inclusive of CAR T-cell therapy.
Inpatient hospital discharges identified on inpatient institutional claims within 90 days after CAR T-cell therapy.
Includes both ED visits without hospital admission (treat and release) and ED visits that resulted in admission.
The Centers for Medicare & Medicaid Services (CMS) cell size suppression policy sets minimum thresholds for the display of CMS data, in which no cell (eg, admissions, discharges, patients, and services) containing a value of 1 to 10 can be reported directly.
Discussion
In this nationally representative RWE study of CAR T-cell therapy in older patients using data of Medicare beneficiaries, CAR T-cell therapy resulted in durable remission in relapsed/refractory DLBCL that appears comparable to outcomes observed among the pivotal phase 2 studies, which included small numbers of older patients, particularly those aged ≥75 years. However, CAR T-cell therapy was not used in >80% of patients who received third-line treatment and beyond, highlighting significant barriers to this treatment among older patients. The reason for not proceeding to CAR T-cell therapy is unknown (ie, if it was due to patient condition, disease progression, or lack of access to cellular therapy for various reasons, including distance to CAR T-cell therapy centers, and family support). Also, patients aged ≥75 years were less frequently treated with CAR T-cell therapy, and EFS was significantly lower among these patients who received CAR T-cell therapy compared with patients aged 65 to 74 years. Although the study confirmed favorable outcomes in older patients for those who received CAR T-cell therapy, the study also demonstrated the limited impact of CAR T-cell therapy in the overall treatment for older patients with relapsed/refractory DLBCL. As the treatment landscape is rapidly evolving, further studies are warranted to evaluate the treatment trend and outcomes of patients who did not receive CAR T-cell therapy.
Multiple studies have evaluated risk factors of failure after CAR T-cell therapy and have identified various patient- and disease-specific factors.15-22 Patient comorbidity, which is a common reason patients are ineligible for prospective clinical trials, has been evaluated in RWE studies and has been associated with less favorable outcomes.21 Recent studies have evaluated various comorbidity indexes that are associated with survival outcomes following CAR T-cell therapy.28-31 Using the Center for International Blood and Marrow Transplant Research cellular therapy registry data, including 1916 patients with relapsed/refractory DLBCL who received commercial CAR T-cell therapy, Greenbaum et al developed a CAR T-cell therapy–specific comorbidity index and reported that a high score is significantly associated with overall mortality from CAR T-cell therapy.30 Other examples include the Chronic Lymphocytic Leukemia Comorbidity Index,32 Hematopoietic Cell Transplantation–Specific Comorbidity Index,33 and Cumulative Illness Rating Scale,31 all confirming the impact of patient comorbidity in CAR T-cell therapy. In this study, we found that CCI was independently associated with shorter EFS and OS. Assessing comorbidity and risk for patients accurately is particularly important in older patients because it may impact the consideration of eligibility for CAR T-cell therapy or identify candidates who may need additional mitigating strategies. More comprehensive geriatric assessment, which includes not just assessment of comorbidity but also other factors, such as activity of daily living, is associated with outcomes in older patients with DLBCL,34 and can potentially reduce the risk of complication from the treatment.35 Assessing prospectively the impact of such geriatric assessment can potentially further guide the eligibility of CAR T-cell therapy in older patients.
Substantial health care use and cost associated with CAR T-cell therapy were observed. The mean total costs during the 90-day period approached $300 000 per patient, with a median cost of ≈$350 000, which is similar or slightly lower to the cost reported for younger patients using 3 different commercial claims database.36 Interestingly, health care service use rates and costs were comparable across age groups, with the exception of the rate of hospitalization within 3 months following receiving CAR T-cell therapy in an outpatient setting, where the cohort aged ≥75 years had a markedly lower hospitalization rate of 30% compared with 54% in those aged 65 to 69 years. Given Medicare reimbursement rates are lower than those of other payer types, the cost presented in the current study appears on the lower end of the real-world cost of CAR T-cell therapy. Several studies have evaluated the cost-effectiveness of CAR T-cell therapy in different lines of treatment among patients with DLBCL.37-41 In these studies, overall cost for CAR T-cell therapy was estimated to be ≈$500 000 to $600 000, and multiple studies supported the cost-effectiveness of CAR T-cell therapy in the long-term; however, these models were compared with standard salvage chemotherapy, which is associated with substantial toxicity and not expected to provide long-term remission. Because of recent advances, treatment for relapsed/refractory DLBCL is becoming more complicated, with novel agents being generally well tolerated in older patients. Some of these treatments may also provide long-term remission,42-45 and can potentially change the cost-effectiveness assumption of CAR T-cell therapy. Particularly in older patients, it is important to balance toxicity and efficacy and tailor the treatment based on the goal of therapy. Further study is warranted to better navigate the various treatment options for older patients; identifying better candidates or treatment strategies will increase the likelihood patients will benefit from the intense and expensive CAR T-cell therapy.
This study has several limitations mainly related to the available data. The date used for this research was from 1 October 2015 through 31 December 2020. The findings of this research may not reflect the most current practice. Given the data source was Medicare claims generated for billing purposes, details of patient characteristics, such as PS, treatment history before Medicare enrollment, and disease characteristics, such as tumor volume, cell of origin, or genetic alterations, which all impact survival outcomes, are not available and thus not considered in the analysis. Also, progression of lymphoma is not captured, and thus EFS used in this study is not equivalent to progression-free survival in other studies. Similarly, we do not have data to inform the reason for inpatient and/or outpatient resource use; these visits may have been related to CAR T-cell therapy–related toxicity management, such as CRS and ICANS, or they may have been other non–lymphoma-related visits. Because different CAR T-cell products can be used in different age groups,46 this may have had an impact on survival outcomes among different age groups. In addition, there are numerous billing codes that are nonspecific to the different CAR T-cell products, thus rendering a product-based analysis infeasible. One could perceive that a better tolerated CAR T-cell therapy may be preferred among older patients, and how the choice of CAR T-cell product impacted outcomes is unknown. Although all patients regardless of follow-up time were included in the survival analyses, patients who died or disenrolled <90 days following the CAR T-cell administration were excluded from health care cost and service use analyses; thus, cost and service use in such patients may be systematically different from those in patients who were followed up >90 days. Last, there could be coding errors in the database, which confer a potential for misclassification of disease status, treatment status, and study outcomes.
In conclusion, this study demonstrated that CAR T-cell therapy provides long-term remission in older patients with relapsed/refractory DLBCL comparable to younger patients; however, the use of current CAR T-cell therapy products seemed to be limited to selected patients, although this may change in the future with next-generation CAR T-cell therapy products. This study indicated an unmet need for more accessible, effective, and tolerable therapy in older patients, especially in patients aged ≥75 years.
Authorship
Contribution: D.C., L.L., L.J.N., and L.C. designed the research; L.L., A.F., J.T., and K.M.K. analyzed the data; and D.C., L.L., J.T., and B.L. wrote the draft; and all authors reviewed and contributed to the final article.
Conflict-of-interest disclosure: D.C. has received honoraria from Eisai and AstraZeneca; J.T. has received research support from ADC Therapeutics; A.F. has received research support from ADC Therapeutics; B.L. has received research support from ADC Therapeutics; K.M.K. has received research support from ADC Therapeutics; L.J.N. has received research support from BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Genmab, Janssen, IGM Biosciences, Novartis, and Takeda; has received honoraria from Genentech/Roche, MEI, and Takeda; and has received other from DSMC, ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead/Kite, Genmab, Janssen, MEI, Morphosys, Novartis, and Takeda; L.L. and L.C. are employees of ADC Therapeutics, with equity and stock options in the company.
Correspondence: Dai Chihara, Department of Lymphoma and Myeloma, The University of Texas MD Anderson Cancer Center, Houston, TX 77030; e-mail: dchihara@mdanderson.org.
References
Author notes
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal