Key Points
Pembrolizumab monotherapy can produce durable responses in a subset of patients with R/R cHL.
Second-course pembrolizumab frequently reinduces a sustained response in patients relapsing from CR.
Abstract
Previous analyses of the phase 2 KEYNOTE-087 (NCT02453594) trial of pembrolizumab monotherapy demonstrated effective antitumor activity with acceptable safety in patients with relapsed or refractory (R/R) classical Hodgkin lymphoma (cHL). However, long-term response durability and outcome of patients who receive a second course after treatment discontinuation after complete response (CR) remain of clinical interest. We present KEYNOTE-087 data after >5 years of median follow-up. Patients with R/R cHL and progressive disease (PD) after autologous stem cell transplantation (ASCT) and brentuximab vedotin (BV; cohort 1), salvage chemotherapy and BV without ASCT (cohort 2), or ASCT without subsequent BV (cohort 3), received pembrolizumab for ≤2 years. Patients in CR who discontinued treatment and subsequently experienced PD were eligible for second-course pembrolizumab. Primary end points were the objective response rate (ORR) using blinded central review and safety. The median follow-up was 63.7 months. ORR was 71.4% (95% confidence interval [CI], 64.8-77.4; CR, 27.6%; partial response, 43.8%). Median duration of response (DOR) was 16.6 months; median progression-free survival was 13.7 months. A quarter of responders, including half of complete responders, maintained a response for ≥4 years. Median overall survival was not achieved. Among 20 patients receiving second-course pembrolizumab, ORR for 19 evaluable patients was 73.7% (95% CI, 48.8-90.8); median DOR was 15.2 months. Any-grade treatment-related adverse events occurred in 72.9% of patients and grade 3 or 4 adverse events occurred in 12.9% of patients; no treatment-related deaths occurred. Single-agent pembrolizumab can induce durable responses, particularly in patients achieving CR. Second-course pembrolizumab frequently reinduced sustained responses after relapse from initial CR.
Introduction
Previous studies have shown that immune checkpoint inhibition targeting programmed death 1 (PD-1) is an effective therapeutic option for patients with relapsed or refractory (R/R) classical Hodgkin lymphoma (cHL) who are ineligible for or progress after autologous stem cell transplantation (ASCT), with or without treatment with brentuximab vedotin (BV).1-4 As a result, the National Comprehensive Cancer Network treatment guidelines recommend the use of the anti–PD-1 checkpoint inhibitor pembrolizumab as an option for second-line treatment in patients ineligible for ASCT or for patients with R/R cHL after ≥2 lines of systemic therapy.5 Pembrolizumab has been approved by the US Food and Drug Administration for the treatment of various solid and hematologic malignancies, including R/R cHL.6 KEYNOTE-087 is a phase 2 study of pembrolizumab in patients with R/R cHL who were ineligible for or progressed after ASCT and/or BV, which demonstrated effective antitumor activity and a favorable safety profile in previous analyses.1,2 With additional follow-up, it is now possible to assess the potential for long-term benefits and the ability to discontinue therapy in patients in complete remission and restart treatment upon subsequent disease progression. To this end, we present data from the KEYNOTE-087 study after >5 years of follow-up.
Methods
Study design
KEYNOTE-087 is a multicenter, single-arm, multicohort, nonrandomized, phase 2 study of pembrolizumab in patients with R/R cHL. The detailed study inclusion and exclusion criteria have been previously described.1,2 Eligible adult patients had R/R cHL that had progressed after the most recent therapy or without a response to the most recent ASCT, with adequate performance status and organ function. All patients provided written informed consent. The study was approved by independent institutional review boards at each study site and conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki.
Patients were enrolled into 3 cohorts: patients whose disease did not respond to or progressed after ASCT and subsequent BV (cohort 1); patients who were unable to achieve a response (ie, complete response [CR] or partial response [PR]) to salvage chemotherapy, did not receive ASCT, and progressed after BV (cohort 2); and patients who did not respond to or whose disease progressed after ASCT and did not receive subsequent BV (cohort 3). All patients in the study received pembrolizumab 200 mg intravenously every 3 weeks for up to 2 years or until disease progression (PD), unacceptable adverse events (AEs), illness preventing further treatment administration, or investigator/patient withdrawal. Patients who achieved CR and had received at least 6 months of treatment with at least 2 doses after CR were allowed to discontinue study treatment before 2 years; those who subsequently experienced PD and did not receive any anticancer treatment because the last dose of pembrolizumab were eligible for a second course of pembrolizumab for up to an additional 17 cycles (∼1 year).
Assessments
Response assessments were performed using positron emission tomography and computed tomography starting at screening. Computed tomography was performed every 12 weeks for subsequent response assessments, and positron emission tomography was performed at weeks 12 and 24 to confirm CR and PD, as clinically indicated. Disease assessments were performed until PD, the start of new anticancer treatment, death, patient withdrawal of consent, or the end of the study. The primary end points were safety and objective response rate (ORR) assessed per the 2007 International Working Group Revised Response Criteria for Malignant Lymphomas (IWG 2007) using blinded independent central review (BICR).7 Secondary end points included ORR per the Lugano 2014 Criteria using BICR,8 duration of response (DOR), progression-free survival (PFS) per the IWG 2007 criteria using BICR, and overall survival (OS). The second-course treatment ORR, DOR, and PFS per the IWG 2007 criteria by investigator assessment, as well as second-course OS, were exploratory. AEs were monitored and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0, until 30 days after the last study dose for all AEs and 90 days after the last study dose for serious AEs.
Statistical analyses
Efficacy and safety were analyzed in all patients who received at least 1 dose of the study treatment. ORR and CR were assessed for the overall population and for each cohort. The ORR using BICR involved the point estimate and 95% 2-sided binomial exact confidence interval (CI) using the Clopper-Pearson method. PFS, OS, and DOR were estimated using the Kaplan-Meier method. Summary statistics were provided for the safety end points. The data cutoff date was 15 March 2021.
Results
Patients
Baseline patient characteristics have been previously presented.1,2 Forty-six percent of patients in the overall population were female. The median age was 35 years (range, 18-76), 46.2% were female, 91.4% were aged <65 years, median prior lines of systemic therapy was 4 (range, 1-12), and 83.3% had prior treatment with BV. Seventy-one patients (33.8%) had primary refractory disease9 and 122 patients (58.1%) were refractory to their last line of therapy. A total of 210 patients were enrolled in the 3 cohorts (69 in cohort 1, 81 in cohort 2, and 60 in cohort 3); 46 patients (21.9%) completed the study treatment, and 164 (78.1%) discontinued the study, most commonly because of PD (41.0%), achievement of CR (13.3%), AEs (8.6%), or physician decisions (4.8%) (supplemental Table 1; available on the Blood website).
Efficacy for the total population and by cohort
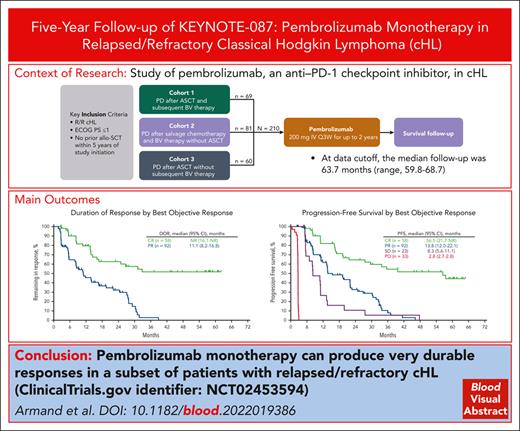
At data cutoff, the median follow-up (time from first study dose to data cutoff) was 63.7 months (range, 59.8-68.7). The ORR for the overall population per the IWG 2007 criteria using BICR was 71.4% (95% CI, 64.8-77.4), with a CR rate of 27.6% and a PR rate of 43.8% (Figure 1). The ORRs for cohorts 1 to 3 were 78.3%, 64.2%, and 73.3%, respectively. Results per the Lugano 2014 criteria using BICR were similar to the ORR per IWG 2007 criteria in the total population (ORR, 73.3% [95% CI, 66.8-79.2] and by cohort (Figure 1).
ORR per IWG 2007 and Lugano 2014 criteria for the overall population and by cohort. NA, not assessed; SD, stable disease.
ORR per IWG 2007 and Lugano 2014 criteria for the overall population and by cohort. NA, not assessed; SD, stable disease.
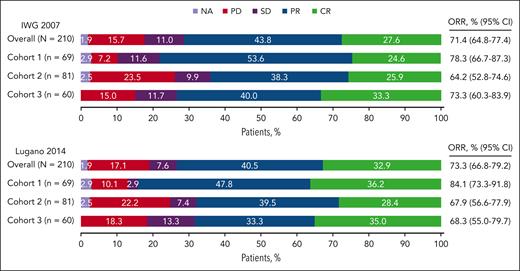
The median DOR for the overall population per IWG 2007 criteria was 16.6 months (95% CI, 11.8-27.1), and 24.8% of the patients, maintained responses for ≥4 years per Kaplan-Meier estimates (Figure 2A). The median DOR for patients in cohorts 1 to 3 was 25.0, 11.1, and 24.4 months, respectively. The median PFS for the overall population was 13.7 months (95% CI, 11.1-19.4), and the 5-year PFS rate was 14.2% (Figure 2B). The median PFS for patients in cohorts 1 to 3 was 16.4, 11.1, and 19.7 months, respectively. The median OS was not reached (NR) for all patients and for patients in each cohort, and the 5-year OS rate for the overall population was 70.7% (Figure 2C).
Kaplan-Meier estimates for the overall population and by cohort. (A) Response duration; (B) PFS; (C) OS.
Kaplan-Meier estimates for the overall population and by cohort. (A) Response duration; (B) PFS; (C) OS.
Efficacy using best objective response
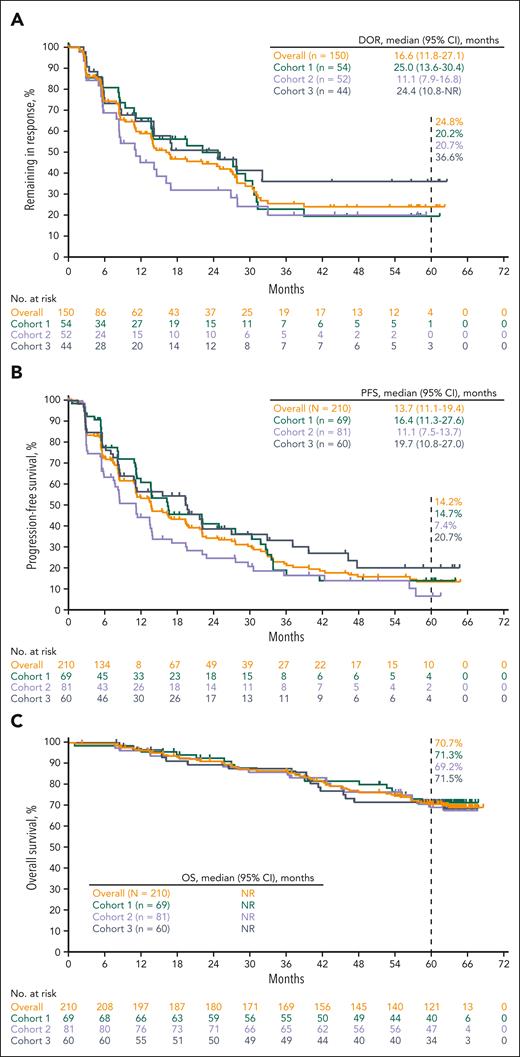
A total of 58 patients across the 3 cohorts achieved CR (cohort 1, n = 17; cohort 2, n = 21; and cohort 3, n = 20) (supplemental Table 2). The median DOR for all patients who achieved CR was not reached (95% CI, 16.1 months-not reached [NR]), and the proportion of patients who remained in complete response after ≥4 years was 51.6%, per Kaplan-Meier estimates (Figure 3A; supplemental Table 2). The median PFS was 56.5 months (95% CI, 21.7-NR), and the 5-year PFS rate was 44.3% (Figure 3B; supplemental Table 2). The median OS was NR, and the 5-year OS rate was 82.8% (Figure 3C; supplemental Table 2).
Kaplan-Meier estimates for the overall population based on the best objective response. (A) Response duration; (B) PFS; (C) OS.
Kaplan-Meier estimates for the overall population based on the best objective response. (A) Response duration; (B) PFS; (C) OS.
Among the 58 patients who achieved CR, 10 patients received allogeneic stem-cell transplant (SCT). Five patients achieved CR with pembrolizumab and then proceeded to allogeneic SCT. The other 5 patients achieved CR to pembrolizumab, subsequently progressed, and then underwent allogeneic SCT. The median DOR for patients who received allogeneic stem cell transplantation was 13.6 months (95% CI, 2.8-NR) (supplemental Figure 1A), and the median PFS was 36.9 months (95% CI, 5.3-57.6) (supplemental Figure 2A). Of the 48 patients who achieved CR and did not undergo subsequent allogeneic SCT, the median DOR was NR (95% CI, 16.8-NR) (supplemental Figure 1B), and the median PFS was 56.5 months (95% CI, 27.6-NR) (supplemental Figure 2B).
For patients with CR who received pembrolizumab for <1 year (21 patients), ≥1 year to <2 years (32 patients), and ≥2 years (5 patients), the median (95% CI) duration of CR was 14.5 (8.5-16.8), not reached (31.2-NR) and 13.8 (5.6-NR) months, respectively; 62.2% in the ≥1 year to <2 years group and 40.0% in the ≥2 years group had a response ≥4 years per Kaplan-Meier estimates (supplemental Table 3)
Forty-six patients who achieved CR per BICR did not receive a second course of pembrolizumab. Of these patients, 13 (28.3%) had an ongoing response at the data cutoff. The median (95% CI) DOR was not reached (26.8-NR); 67.4% of patients had a response of ≥4 years per Kaplan-Meier estimate (supplemental Table 4).
Of the 92 patients who achieved PR (37 in cohort 1, 31 in cohort 2, and 24 in cohort 3), 2 patients underwent SCT, 15 patients started new anticancer treatment, 4 patients were lost to follow-up, and 5 patients had disease progression or died after ≥2 missed visits. The median DOR for all patients who achieved PR was 11.1 months (95% CI, 8.2-16.8), and the proportion of patients who remained in PR for ≥3 years was 3.0%, per Kaplan-Meier estimates (Figure 3A; supplemental Table 2). Among the patients who achieved PR, the median PFS was 13.8 months (95% CI, 12.0-22.1) and the 3-year PFS rate was 10.3% (Figure 3B; supplemental Table 2). The median OS for all patients who achieved PR was not reached (95% CI, NR-NR), and the 5-year OS rate was 75.5% (Figure 3C; supplemental Table 2).
Among the 23 patients who achieved stable disease, the median PFS was 8.3 months (95% CI, 5.6-11.1) and the 3-year PFS rate was 5.3% (Figure 3B). The median OS was not reached (95% CI, 26.8-NR), and the 5-year OS rate was 53.7% (Figure 3C). Among the 33 patients with disease progression, the median PFS was 2.8 months (95% CI, 2.7-2.8) (Figure 3B). The median OS was 62.9 months (95% CI, 36.4-NR), and the 5-year OS rate was 50.6% (Figure 3C).
Efficacy in patients who received a second-course treatment per investigator review
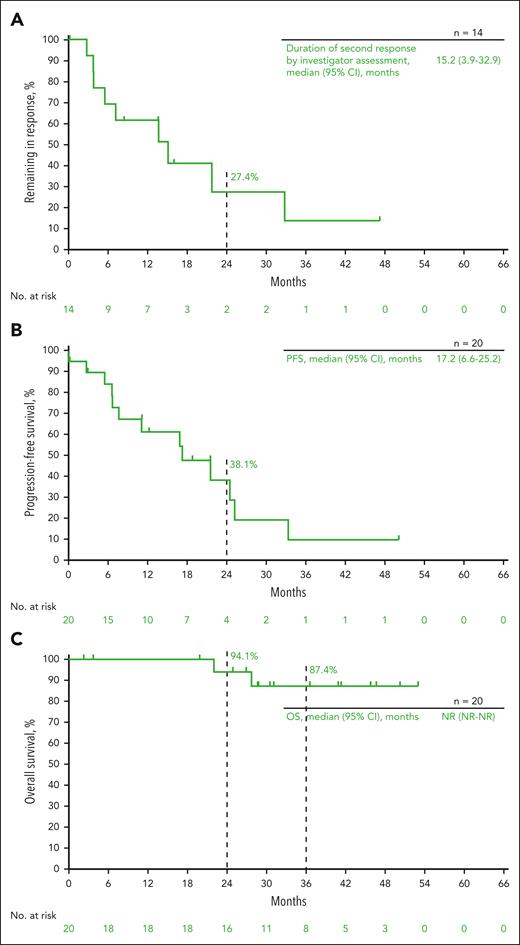
Twenty patients who achieved CR per the investigator’s assessment (12 of these patients also achieved CR using BICR) and experienced disease progression received second-course pembrolizumab (10 in cohort 1, 7 in cohort 2, and 3 in cohort 3) (supplemental Figure 3). The median duration of the initial response before second-course treatment was 27.2 months (95% CI, 16.8-30.2). Ten patients completed 17 additional cycles of pembrolizumab, and 1 patient was still in the second treatment at the data cutoff. One patient was not included in the response analysis of patients who received second-course pembrolizumab because response data were not available at the time of data cutoff. The ORR per the IWG 2007 criteria for the 19 evaluable patients was 73.7% (95% CI, 48.8-90.8), with 7 patients (36.8%) achieving CR and 7 patients (36.8%) achieving PR (Table 1). The ORR for patients in cohorts 1 to 3 per IWG 2007 criteria was 77.8%, 85.7%, and 33.3%, respectively. The median DOR for all patients who received the second-course treatment was 15.2 months (95% CI, 3.9-32.9) (Figure 4A). Of patients who experienced a CR to second-course treatment (n = 7), the median DOR was 21.8 months (95% CI, 7.3-NR). The median PFS was 17.2 months (95% CI, 6.6-25.2), and the 2-year PFS rate was 38.1% (Figure 4B). The median OS was NR, and the 2-year and 3-year OS rates were 94.1% and 87.4%, respectively (Figure 4C). Two patients received a subsequent allogeneic stem cell transplantation after second-course pembrolizumab.
Summary of overall response per the IWG 2007 criteria by investigator assessment in patients who received second-course treatment and had response data at data cutoff
| . | Cohort 1 (n = 9) . | Cohort 2 (n = 7) . | Cohort 3 (n = 3) . | Total (n = 19) . |
|---|---|---|---|---|
| ORR, % (95% CI) | 77.8 (40.0-97.2) | 85.7 (42.1-99.6) | 33.3 (0.8-90.6) | 73.7 (48.8-90.8) |
| BOR, n (%) | ||||
| CR | 1 (11.1) | 6 (85.7) | 0 (0) | 7 (36.8) |
| PR | 6 (66.7) | 0 (0) | 1 (33.3) | 7 (36.8) |
| SD | 1 (11.1) | 0 (0) | 2 (66.7) | 3 (15.8) |
| PD | 1 (11.1) | 1 (14.3) | 0 (0) | 2 (10.5) |
| . | Cohort 1 (n = 9) . | Cohort 2 (n = 7) . | Cohort 3 (n = 3) . | Total (n = 19) . |
|---|---|---|---|---|
| ORR, % (95% CI) | 77.8 (40.0-97.2) | 85.7 (42.1-99.6) | 33.3 (0.8-90.6) | 73.7 (48.8-90.8) |
| BOR, n (%) | ||||
| CR | 1 (11.1) | 6 (85.7) | 0 (0) | 7 (36.8) |
| PR | 6 (66.7) | 0 (0) | 1 (33.3) | 7 (36.8) |
| SD | 1 (11.1) | 0 (0) | 2 (66.7) | 3 (15.8) |
| PD | 1 (11.1) | 1 (14.3) | 0 (0) | 2 (10.5) |
One patient was not included because response data were not available at the time of data cutoff.
BOR, best overall response; SD, stable disease.
Kaplan-Meier estimates for patients who received second-course treatment. (A) Duration of the second response; (B) PFS; (C) OS.
Kaplan-Meier estimates for patients who received second-course treatment. (A) Duration of the second response; (B) PFS; (C) OS.
Safety
Adverse events occurred in 205 patients (97.6%) and 153 patients (72.9%) experienced treatment-related AEs. The most commonly reported treatment-related AEs occurring in >10% of patients were hypothyroidism (14.3%), pyrexia (11.4%), fatigue (11.0%), and rash (11.0%) (Table 2; supplemental Table 5). Grade 3 or 4 treatment-related AEs occurred in 27 patients (12.9%) and the AEs most commonly reported (occurring in ≥2 patients) were neutropenia (2.4%), pericarditis (1.0%), and diarrhea (1.0%). Fourteen patients (6.7%) discontinued the study because of treatment-related AEs. No treatment-related deaths were reported. Treatment-related AEs according to the cohort are presented in supplemental Table 3.
Treatment-related adverse events
| TRAEs . | n (%) for overall population, N = 210 . |
|---|---|
| Any-grade TRAE | 153 (72.9) |
| TRAEs ≥5% | |
| Hypothyroidism | 30 (14.3) |
| Pyrexia | 24 (11.4) |
| Fatigue | 23 (11.0) |
| Rash | 23 (11.0) |
| Diarrhea | 17 (8.1) |
| Headache | 16 (7.6) |
| Nausea | 15 (7.1) |
| Arthralgia | 13 (6.2) |
| Cough | 13 (6.2) |
| Pruritus | 13 (6.2) |
| Infusion-related reaction | 11 (5.2) |
| Neutropenia | 11 (5.2) |
| Grade 3 or 4 TRAEs | 27 (12.9) |
| Grade 3 or 4 TRAEs in ≥2 patients | |
| Neutropenia | 5 (2.4) |
| Pericarditis | 2 (1.0) |
| Diarrhea | 2 (1.0) |
| TRAEs . | n (%) for overall population, N = 210 . |
|---|---|
| Any-grade TRAE | 153 (72.9) |
| TRAEs ≥5% | |
| Hypothyroidism | 30 (14.3) |
| Pyrexia | 24 (11.4) |
| Fatigue | 23 (11.0) |
| Rash | 23 (11.0) |
| Diarrhea | 17 (8.1) |
| Headache | 16 (7.6) |
| Nausea | 15 (7.1) |
| Arthralgia | 13 (6.2) |
| Cough | 13 (6.2) |
| Pruritus | 13 (6.2) |
| Infusion-related reaction | 11 (5.2) |
| Neutropenia | 11 (5.2) |
| Grade 3 or 4 TRAEs | 27 (12.9) |
| Grade 3 or 4 TRAEs in ≥2 patients | |
| Neutropenia | 5 (2.4) |
| Pericarditis | 2 (1.0) |
| Diarrhea | 2 (1.0) |
TRAE, treatment–related adverse event.
Among the patients who received second-course pembrolizumab (n = 20), 19 (95.0%) experienced a treatment-related AE and the most common AEs (≥15%) were fatigue (20.0%), diarrhea, muscle spasms, and rash (15.0% each) (supplemental Table 6). Seven grade 3 treatment-related AE occurred in 6 patients; no grade 4 or 5 events occurred. None of the patients discontinued second-course pembrolizumab or died because of treatment-related AEs.
Discussion
Previous studies have demonstrated robust antitumor activity and manageable safety of pembrolizumab in R/R cHL.1,2,4,10 The superior PFS associated with this therapy compared with BV was demonstrated in a phase 3 randomized, open-label KEYNOTE-204 study of pembrolizumab or BV in patients with R/R cHL, who were refractory to or ineligible for ASCT (N = 304).10 The present results confirmed that the safety profile of pembrolizumab was maintained with additional follow-up, with no new toxicity signals. In addition, this study allowed us to address the important clinical question of response durability in patients with R/R cHL. With long-term follow-up, we report that a subset of patients, comprising a quarter of responders and half of complete responders, can obtain durable benefits (with more than 4 years of response duration) regardless of previous receipt and timing of BV. Although it is still too early to speculate on an even longer time horizon, the shape of the DOR curves (Figures 2A and 3A) suggests the provocative possibility that these responders may remain in remission for an extended period of time, even beyond the currently reported 5-year time point.
In this context, it is important to understand the predictors of long-term benefits. Consistent with previous studies,3 the best predictor of long-term benefit to date is the depth of radiographic response. In fact, all patients in the long-term response group (>4 years) were complete responders, and conversely, among patients achieving CR, more than half remained in CR after 4 years. This provides actionable information for patients considering additional therapies, such as stem cell transplantation during pembrolizumab-induced remission. Our results also raise the question of whether the CR rate may be a useful surrogate of the long-term benefit of PD-1 blockade–based combination therapies, although this hypothesis requires validation. Conversely, patients who did not achieve CR with pembrolizumab had poorer outcomes, with very few long-term remissions maintained. This raises the question of whether these patients are best served by consolidation strategies. Of note, the outcomes of patients in our cohort who received allogeneic stem cell transplantation appeared less favorable than those in the larger series11; this likely reflects the small number of patients concerned and limited follow-up.
An additional benefit of the long-term follow-up of KEYNOTE-087 patients is the ability to report outcomes for patients who enter CR and whose disease subsequently progresses. Although the numbers were small and the follow-up for the second course was short, our results suggest that retreatment with pembrolizumab provides a high probability (74%) of achieving a second remission, making this an excellent choice of therapy for such patients; they also support the ability to stop treatment before 2 years in patients achieving CR with first-course pembrolizumab. However, the DOR curve (Figure 4A) suggests that a few patients in this setting will maintain remission beyond ∼3 years, which may be relevant to decisions regarding stem cell transplantation consolidation. In addition, the promising antitumor activity of the second-course therapy raises the possibility that combining pembrolizumab with other agents in this setting (eg, chemotherapy) may provide another important salvage option.
Similarly, favorable results were preliminarily reported with long-term follow-up of the PD-1 inhibitor nivolumab in a phase 2 CheckMate-205 study.12 One notable difference between the 2 studies is that patients on CheckMate-205 continued treatment until PD, whereas patients on KEYNOTE-087 received a maximum of 2 years of treatment (for first-course therapy). Given the overall similarity of long-term PFS and DOR between the studies and acknowledging the perils of cross-trial comparisons, one may reasonably conclude that there is no apparent benefit of continuing PD-1 blockade treatment beyond 2 years in patients with R/R cHL.
In conclusion, the current analysis reports long-term data on patients with R/R cHL treated with PD-1 blockade. This confirmed the strong activity of pembrolizumab in this setting, without any new safety concerns. More importantly, it demonstrates the ability of some patients (approximately one-quarter of responders and one-half of complete responders) to maintain durable remission, the importance of radiographic depth of response as a predictor of long-term benefit, and the feasibility of stopping treatment for patients in CR and resuming following disease progression. Such information may be valuable in guiding therapy choices for patients with R/R cHL, as well as for informing decisions regarding possible consolidation of remission for responders.
Acknowledgments
The authors thank the patients and their families and all investigators and site personnel integral to the completion of the study. The authors also thank May Tang (employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ) for her contribution to the statistical analysis of the manuscript. Medical writing and editorial assistance were provided by Dominic Singson and Matthew Grzywacz of ApotheCom (Yardley, PA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc, Rahway, NJ.
This work was supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc, Rahway, NJ.
Authorship
Contribution: P.A., P.B., B.v.T., M.A.S., P.M., and C.H.M. contributed to conception, design, or planning of the study; P.A., N.A.J., J.R., V.R., D.M., T.P.V., A.T., B.v.T., A.F.H., S.C., and C.H.M. contributed to acquisition of the data; P.L.Z., V.R., M.A.S., J.L., E.K., S.C., P.M., and C.H.M. contributed to analysis of the data; P.A., P.L.Z., H.J.L., J.R., V.R., T.P.V., B.v.T., M.A.S., A.F.H., E.K., S.C., P.M., and C.H.M. contributed to interpretation of the results; H.J.L., P. B., S.C., and C.H.M. contributed to drafting the manuscript; P.A., P.L.Z., H.J.L., N.A.J., J.R., V.R., D.M., T.P.V., A.T., B.v.T., M.A.S., A.F.H., J.L., S.C., P.M., and C.H.M. contributed to critically reviewing or revising the manuscript for important intellectual content; and all authors approved the final version of the manuscript for submission.
Conflict-of-interest disclosure: P.A. reports grants or contracts to his institution from Merck, BMS, Adaptive, Genentech, IGM, and AstraZeneca within the last 24 months and Affimed, Tensha, Otsuka, and Sigma Tau; research funding from Kite within the last 24 months; consulting fees from Merck, BMS, ADC Therapeutics, GenMab, Enterome, Genentech/Roche, ATB Therapeutics, and Foresight within the last 24 months and from Pfizer, Affimed, Infinity, ADC Therapeutics, Celgene, Morphosys, Daiichi Sankyo, Miltenyi, Tessa, GenMab, C4, Enterome, Regeneron, Epizyme, and AstraZeneca; and honoraria from Merck and BMS. P.L.Z. reports honoraria from Roche, Takeda, Gilead, Sanofi, Incyte, Novartis, Merck, BMS, AstraZeneca, and Kyowa Kirin. H.J.L. reports grants or contracts from BMS, Seagen, Oncternal, Merck, and Takeda; consulting fees from Century Therapeutics; and honoraria from Aptitude Health and the Korean Society of Cardio-Oncology. N.A.J. reports consulting fees from Merck, AbbVie, BeiGene, Roche, BMS, and Kite; and honoraria from Roche, AbbVie, and BeiGene. V.R. reports advisory board roles with Gilead, Infinity, MSD, BMS, Nanostring, Incyte, Roche, and AstraZeneca; and research funding from Astex, Argen-X, and GSK. D.M. reports honoraria from MSD and BMS. T.P.V. reports grants or contracts from Merck, Takeda, Amgen, Pfizer, and Dr Reddy’s; consulting fees from Takeda, Roche, Genesis Pharma, and Novartis; honoraria from Takeda, Roche, Genesis Pharma, Merck, Novartis, Amgen, GSK, AbbVie, Integris, and AstraZeneca; and stock or stock options in Merck. A.T. reports grants or contracts from Chugai Pharmaceutical, Kyowa Kirin, Perseus Proteomics, Pfizer Japan, and Novartis Pharma K.K.; and honoraria from Chugai Pharmaceutical, Takeda Pharmaceutical, Nippon Shinyaku, Ono Pharmaceutical, and Eisai. B.v.T. reports grants or contracts from Novartis, Takeda, and Merck Sharp & Dohme; consulting fees from Allogene, BMS/Celgene, Cerus, Incyte, Miltenyi, Novartis, Pentixafarm, Roche, Amgen, Pfizer, Takeda, Merck Sharp & Dohme, and Gilead Kite; honoraria from AstraZeneca, Novartis, Roche Pharma AG, Takeda, and Merck Sharp & Dohme; and support for attending meetings and/or travel from AbbVie, AstraZeneca, Kite-Gilead, Merck Sharp & Dohme, Takeda, and Novartis. M.A.S. reports grants or contracts from BMS, Bayer, AbbVie, and AstraZeneca; and consulting fees from AstraZeneca. A.F.H. reports grants or contracts from BMS, Genentech, Merck, Seattle Genetics, AstraZeneca, Karyopharm, Kite Pharma, Gilead Sciences, AstraZeneca, and ADC Therapeutics; and consulting fees from BMS, Genentech, Merck, Seattle Genetics, AstraZeneca, Karyopharm, ADC Therapeutics, Takeda, Tubulis, Regeneron, Genmab, and Pfizer. J.L. reports employment at Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, and is a share/stockholder or has stock options in Merck & Co Inc, Rahway, NJ. E.K. reports employment at Merck Sharp & Dohme LLC, a subsidiary of Merck & Co Inc, Rahway, NJ, at the time of this analysis. S.C. reports employment at Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, and is a share/stockholder or has stock options in Merck & Co Inc, Rahway, NJ. P.M. reports employment at Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, and is a share/stockholder or has stock options in Merck & Co, Inc, Rahway, NJ. C.H.M. reports research funding from Merck & Co, Inc. The remaining authors declare no competing financial interests.
Correspondence: Philippe Armand, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215;.
References
Author notes
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials to conduct legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, have a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data-sharing website (available at http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. A committee of subject matter experts of MSD will review feasible requests to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with the MSD before data access is granted. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country- or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts. After approval of the statistical analysis plan and execution of a data-sharing agreement, the MSD will either perform the proposed analyses and share the results with the requestor, or construct biomarker covariates and add them to a file with clinical data that will be uploaded to an analysis portal, so that the requestor can perform the proposed analyses.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal