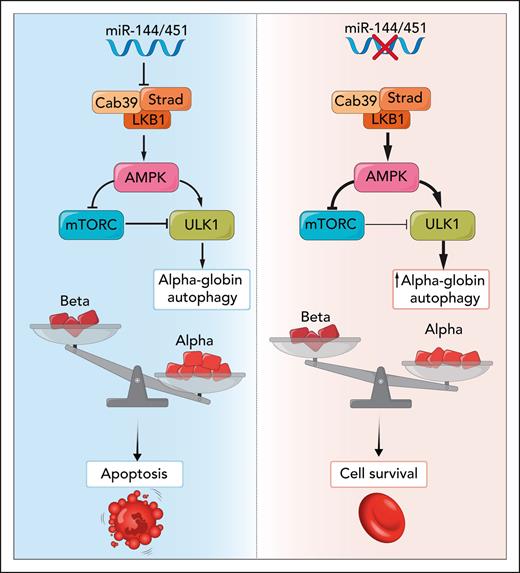
In this issue of Blood, Keith and colleagues show the miR-144/451 locus is a genetic modifier of β-thalassemia by regulating removal of free α-globin via the autophagy kinase ULK1 (Unc-51–like autophagy-activating kinase 1).1 miR-144/451 is the highest expressed miR locus in terminal erythroid differentiation, and its disruption in a β-thalassemia mouse model releases inhibition of the Cab39/Strad/LKB1 complex. Increased expression of Cab39/Strad/LKB1 activates adenosine monophosphate–activated protein kinase (AMPK), sometimes referred to as the “cellular fuel gauge.” Increased AMPK signaling reduces mammalian target of rapamycin complex 1 (mTORC1)-mediated repression of ULK1 and directly activates ULK1 to give it super strength to degrade free α-globin, which, if it accumulates, is so toxic that it triggers apoptosis (see figure). The role of AMPK in this pathway suggests existing drugs that increase AMPK activity2 could be repurposed as part of a β-thalassemia combination therapy.
In β-thalassemia, during terminal erythroid maturation, excess α-globin chains are the chief culprit in causing ineffective erythropoiesis and hemolysis. Disruption of miR-144/451 increases Cab39/Strad/LKB1 activation of AMPK and increases autophagy of α-globin via activation of the ULK1 autophagy kinase, improving cell survival. Professional illustration by Somersault18:24.
In β-thalassemia, during terminal erythroid maturation, excess α-globin chains are the chief culprit in causing ineffective erythropoiesis and hemolysis. Disruption of miR-144/451 increases Cab39/Strad/LKB1 activation of AMPK and increases autophagy of α-globin via activation of the ULK1 autophagy kinase, improving cell survival. Professional illustration by Somersault18:24.
β-Thalassemia, caused by a deficit in the production of the β-globin component of hemoglobin, is one of the most common monogenic diseases. In most patients, clinical management still largely depends on supportive treatment with red blood cell transfusions and iron chelation.3 Hydroxyurea is used to increase γ-globin expression as this fetal β-like globin combines with free α-globin to form fetal hemoglobin, increasing functional hemoglobin levels and reducing toxic levels of unpaired α-globin. Until recently, allogenic bone marrow transplantation remained the only curative treatment; however, its usefulness is largely limited to a minority of patients who have HLA-matched sibling donors.4
Most emerging therapies for β-thalassemia aim to supplement reduced β-globin levels, by either inducing expression of the γ-globin genes or replacing the damaged or missing β-globin genes using lentiviral vectors.5 One particularly elegant strategy is to delete an erythroid-specific enhancer of BCL11A, one of the main γ-globin repressors,6 to reduce its expression in erythroblasts.
Although increasing β-like globin leads to increased functional hemoglobin, it is important not to overlook its role in reducing the excess of the free α-globin chains, which are the primary culprit in β-thalassemia pathology as they cause ineffective erythropoiesis. Mae West was wrong in the case of α-globin: too much of a good thing is not always wonderful. Reduction of α-globin levels as a therapy for β-thalassemia has been previously suggested (eg, by removal of an α-globin enhancer7 to reduce α-globin protein levels). Evidence from clinical studies shows a reduction of α-globin expression to 75% to 25% of normal is effective, safe, and tolerable and provides sustainable beneficial effects in patients with β-thalassemia.8 Natural modifiers, such as coinherited α-thalassemia, can modify a genotype associated with transfusion-dependent β-thalassemia into a clinical phenotype of β-thalassemia intermedia, a milder condition. Conversely, inheriting triplicated copies of the α-globin gene can worsen the outcome in an individual who is a β0-thalassemia carrier, such that the individual manifests a clinical presentation of β-thalassemia intermedia. Subtle changes in the globin balance really do make a difference.
Previous studies have shown the severity of β-thalassemia phenotypes may be modulated by mTORC1 via ULK1-mediated autophagy and that ULK1 activity is regulated via AMPK.9 It is also known that miR-144/451 inhibits AMPK in erythroid cells.10 The study by Keith et al in this issue shows, for the first time, by genetic disruption of the miR-144/451 locus, the beneficial impact of this pathway in β-thalassemia. In contrast to the detrimental effect of disruption of miR-144/451 activation in normal erythroid cells, increasing ULK1-mediated autophagy in the context of β-thalassemia ameliorates the phenotypic severity. The reduction in free α-globin, shown by electron microscopy in Figure 2, is especially striking.
The effects of loss of miR-144/451 are pleiotropic, as there is also reduction in the transferrin receptor, leading to iron restriction, which may also improve β-thalassemia phenotypes. However, it would be important to determine the effect of reduced iron uptake by the erythroid pool on systemic iron loading. In addition, further work is needed to establish the link between miR-144/451 and AMPK activation in human erythroid cells, as this was not recapitulated in cultured erythroblasts.
The current study offers the exciting potential to investigate the use of existing drugs that modulate AMPK activity as a novel therapeutic modality in β-thalassemia by removing damaging α-globin chains at the protein level. This approach has the potential to form part of a combination therapy along with the therapeutic activation of γ-globin and drugs, such as luspatercept, thought to improve ineffective erythropoiesis by modulating apoptosis. As genome therapy is unlikely to be widely available for most patients with β-thalassemia in the medium term, drug approaches are needed, and sending excess free α-globin chains west may offer a useful component of future therapeutic approaches.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal