In this issue of Blood, Courbon et al demonstrate that inflammation stimulates production of C-terminal fibroblast growth factor 23 (cFGF23) fragments that downregulate hepcidin and mitigate iron deficiency.1
Why would a cell engage in an energy-intensive and a seemingly wasteful exercise of producing a peptide hormone only to immediately degrade it intracellularly and secrete the resulting fragments into circulation? And why do inflammation and iron deficiency recruit such a process to produce large amounts of fragments of a phosphate-regulating hormone?
FGF23 is a bone-derived peptide hormone that regulates mineral homeostasis via multiple negative endocrine feedback loops.2 FGF23 production is stimulated by increases in 1,25-dihydroxyvitamin D (1,25D), parathyroid hormone (PTH), serum calcium, and kidney-derived glycerol-3-phosphate, which is the effector of phosphate sensing by the proximal tubule of the kidney.2,3 Closing the feedback loops, each of these stimuli induce secretion of full-length FGF23 by bone, which travels to the kidney to stimulate renal phosphate excretion, reduce 1,25D concentrations, and effectuate downstream changes in calcium and PTH.2
Inflammation and iron deficiency also activate FGF23 production, but these stimuli couple increases in FGF23 transcription to increased intracellular cleavage of newly translated FGF23 into C-terminal and N-terminal fragments (see figure).4 This coordinated activation of FGF23 production and cleavage results in no change in full-length FGF23 levels but markedly increases circulating concentrations of FGF23 fragments that have no effect on phosphate homeostasis.4 The discovery of this peculiar phenomenon was facilitated by the serendipitous development of C-terminal FGF23 (cFGF23) assays that detect full-length FGF23 and its C-terminal fragments.5 This assay complements intact FGF23 assays (iFGF23) that exclusively detect the full-length hormone. Since iFGF23 levels and thus serum phosphate are normal in patients with inflammation and iron deficiency, markedly elevated concentrations of cFGF23 fragments that are detected by cFGF23 assays are the only clinical footprint of excessive FGF23 production and cleavage.6
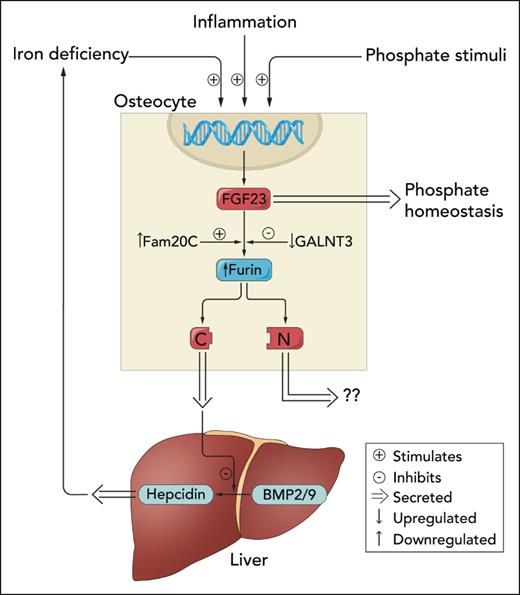
Regulation of iron or phosphate homeostasis by C-terminal vs full-length FGF23. Inflammation, iron deficiency, and phosphate-related stimuli activate FGF23 transcription in osteocytes. Newly translated FGF23 protein is either glycosylated by GALNT3 and secreted into circulation to regulate phosphate homeostasis or phosphorylated by Fam20C and cleaved by furin. Courbon et al demonstrate that inflammation also increases expression of Fam20C and Furin and decreases GALNT3 expression. C-terminal FGF23 fragments are secreted by osteocytes and travel to the liver to antagonize BMP-mediated increases in hepcidin production. Presumably, N-terminal FGF23 fragments are also secreted, but their potential biological functions are currently unknown. Professional illustration by Somersault18:24.
Regulation of iron or phosphate homeostasis by C-terminal vs full-length FGF23. Inflammation, iron deficiency, and phosphate-related stimuli activate FGF23 transcription in osteocytes. Newly translated FGF23 protein is either glycosylated by GALNT3 and secreted into circulation to regulate phosphate homeostasis or phosphorylated by Fam20C and cleaved by furin. Courbon et al demonstrate that inflammation also increases expression of Fam20C and Furin and decreases GALNT3 expression. C-terminal FGF23 fragments are secreted by osteocytes and travel to the liver to antagonize BMP-mediated increases in hepcidin production. Presumably, N-terminal FGF23 fragments are also secreted, but their potential biological functions are currently unknown. Professional illustration by Somersault18:24.
In this issue of Blood, Courbon et al elegantly address why inflammation activates the FGF23 production-cleavage cycle in bone. The authors confirm that increased transcription and intracellular cleavage of FGF23 by furin in osteocytes is the source of the excess C-terminal FGF23 fragments that are produced in response to inflammation (see figure). Although bone marrow stromal cells were another major source of basal FGF23 production, inflammation did not alter their FGF23 production. To elucidate potential actions of C-terminal FGF23 fragments, the authors investigated the effects of inflammation on hepcidin production in a series of mouse models with deficient or excessive production of C-terminal FGF23 fragments. Compared with wild-type mice, inflammation-induced iron deficiency was exacerbated by further increases in hepcidin levels in mice that were unable to augment production of C-terminal FGF23 fragments due to osteocyte-specific deletion of FGF23 or furin. Conversely, injection or transgenic overexpression of C-terminal FGF23 fragments in inflamed mice decreased hepcidin levels and mitigated iron deficiency.
The authors confirmed that the effects on hepcidin were specific to C-terminal FGF23 fragments vs full-length FGF23 by demonstrating no changes in hepcidin or iron stores in a mouse model with isolated increases in full-length FGF23. As the underlying molecular mechanism, the authors reported that C-terminal FGF23 fragments antagonize bone morphogenic protein (BMP) 2/9-driven hepcidin production (see figure), perhaps by preventing BMP2/9 from binding to BMP receptors. In summary, breaking open full-length, phosphate-regulating FGF23 reveals a smaller, iron-regulating C-terminal fragment. One parent peptide, 2 separate hormones governing 2 distinct pathways.
Competing posttranslational modifications determine whether nascent FGF23 is cleaved or secreted intact. Glycosylation of FGF23 by GALNT3 protects FGF23 from cleavage by furin and results in secretion of full-length hormone, whereas phosphorylation by Fam20C earmarks FGF23 for cleavage.4 Courbon et al found that inflammation rapidly increases expression of Fgf23, Fam20C, and Furin while rapidly decreasing Galnt3 expression (see figure). Although the authors did not report on the molecular mechanism of how inflammation orchestrates this program of gene expression that culminates in increased production of FGF23 fragments, prior studies suggest that stabilizing or increasing expression of hypoxia-inducible factor (HIF) 1α may play a central role.7 Prolyl hydroxylase inhibitors potentiate the effects of HIF1α and are known to decrease hepcidin.8 Whether increased production of C-terminal FGF23 fragments contributes to hepcidin suppression by factors that stabilize HIF1α is worthy of additional research.
Future studies should also address how this complex system responds to competing stimuli, for example, when inflammation and iron deficiency demand production of C-terminal FGF23 fragments at the same time that high-phosphate diet calls for full-length FGF23. Likewise, future studies should investigate how administration of intravenous ferric carboxymaltose converts patients with iron deficiency anemia from a state of high C-terminal FGF23 fragments to a state of high full-length FGF23 that often causes severe hypophosphatemia.6,9 Based on the work by Courbon et al, it seems likely that ferric carboxymaltose somehow reduces FGF23 cleavage by short-circuiting the cellular controls that dictate the relative production of full-length vs FGF23 fragments.
Chronic kidney disease is another state of reduced FGF23 cleavage in which circulating concentrations of full-length FGF23 progressively increase while C-terminal fragments gradually decline.2,4 In this setting, reduced FGF23 cleavage is considered to be an adaptive mechanism for osteocytes to augment production of full-length FGF23 to maintain normal serum phosphate in the face of severe reductions in kidney function. However, the data presented by Courbon et al suggest that this process might also be maladaptive if reduced production of C-terminal FGF23 fragments contributes to worsening anemia of kidney disease by failing to suppress hepcidin.
The new data from Courbon et al will have far-reaching effects on our understanding of iron and phosphate homeostasis and anemia of chronic disease, including in patients with chronic kidney disease. They also suggest potential therapeutic applications. For example, perhaps exogenous cFGF23 fragments or small molecules that harness their ability to inhibit BMP-mediated production of hepcidin could become novel candidates to improve the lives of patients with anemia of chronic disease.
Conflict-of-interest disclosure: M.W. has equity interests in Akebia, Unicycive, and Walden and has served as a consultant for Bayer, Enyo, Jnana, Kissei, Launch, Pharmacosmos, Reata, and Torii.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal