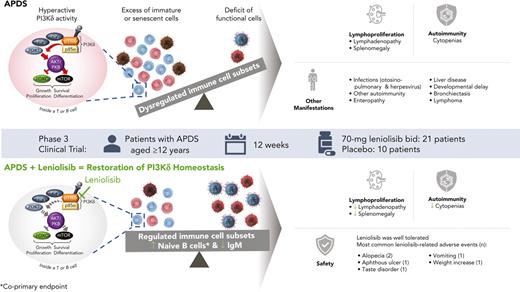
Key Points
The oral PI3Kδ inhibitor leniolisib reduced lymphadenopathy and normalized immune cell subsets in patients with APDS, an inborn error of immunity.
Leniolisib was well tolerated in patients with APDS, with mostly grade 1 AEs and no serious AEs related to study treatment.
Abstract
Activated phosphoinositide 3-kinase delta (PI3Kδ) syndrome (APDS) is an inborn error of immunity with clinical manifestations including infections, lymphoproliferation, autoimmunity, enteropathy, bronchiectasis, increased risk of lymphoma, and early mortality. Hyperactive PI3Kδ signaling causes APDS and is selectively targeted with leniolisib, an oral, small molecule inhibitor of PI3Kδ. Here, 31 patients with APDS aged ≥12 years were enrolled in a global, phase 3, triple-blinded trial and randomized 2:1 to receive 70 mg leniolisib or placebo twice daily for 12 weeks. Coprimary outcomes were differences from baseline in the index lymph node size and the percentage of naïve B cells in peripheral blood, assessed as proxies for immune dysregulation and deficiency. Both primary outcomes were met: the difference in the adjusted mean change (95% confidence interval [CI]) between leniolisib and placebo for lymph node size was −0.25 (−0.38, −0.12; P = .0006; N = 26) and for percentage of naïve B cells, was 37.30 (24.06, 50.54; P = .0002; N = 13). Leniolisib reduced spleen volume compared with placebo (adjusted mean difference in 3-dimensional volume [cm3], −186; 95% CI, −297 to −76.2; P = .0020) and improved key immune cell subsets. Fewer patients receiving leniolisib reported study treatment-related adverse events (AEs; mostly grades 1-2) than those receiving placebo (23.8% vs 30.0%). Overall, leniolisib was well tolerated and significant improvement over placebo was notable in the coprimary endpoints, reducing lymphadenopathy and increasing the percentage of naïve B cells, reflecting a favorable impact on the immune dysregulation and deficiency seen in patients with APDS. This trial was registered at www.clinicaltrials.gov as #NCT02435173.
Introduction
Activated phosphoinositide 3-kinase delta (PI3Kδ) syndrome (APDS) is an inborn error of immunity resulting from pathogenic heterozygous variants in either of the genes encoding the PI3Kδ heterodimer. Gain-of-function variants in PIK3CD encoding the catalytic subunit p110δ cause APDS1, whereas loss-of-function variants in PIK3R1 encoding the regulatory subunit p85α cause APDS2.1-3 Interaction of the 2 subunits is essential for heterodimer function and stability.4 Disruption of this interaction or loss of p85α-mediated inhibition because of pathologic variants in PIK3CD or PIK3R1 result in hyperactive PI3Kδ signaling.1,2,4 This hyperactive signaling results in comparable immunologic consequences in both APDS1 and APDS2.4-6
APDS is a complex immune deficiency with striking immune dysregulation and diverse clinical presentation (supplemental Figure 1; available on the Blood website).7,8 This heterogenous presentation is caused in part by dysregulated B and T cells.9,10 Proper lymphocyte development and function depends on a tightly balanced PI3Kδ pathway: enzyme activity must be variably increased and dampened at different time points to allow for FOXO-dependent signaling.11 Constitutively active PI3Kδ, as occurs in patients with APDS, results in disturbed immune cell development and function.1,2
Hallmarks of the clinical presentation are recurrent sinopulmonary infections and prolonged or intermittent herpesvirus viremia.5-8 These infections are due to immunoglobulin dysfunction or related to increases in immature/dysfunctional B and T lymphocytes at the expense of mature/functional cells.12-14 Patients with APDS often have an inverted CD4+/CD8+ T-cell ratio, which is primarily driven by an increase in terminal differentiation.1,5,6 Naïve T cells decrease, and patients often have an increase in cytotoxic CD8+ effector memory and terminally differentiated effector memory cells at the expense of long-lived central memory cells.1,2,5,6 These cells also tend to resemble highly inflammatory senescent or exhausted CD8+ T cells.1,2,12 The shifts in T-cell subsets result in ineffective responses to infections, particularly chronic infections such as Epstein-Barr virus (EBV) and cytomegalovirus (CMV).12-14 In the B-cell compartment, immature transitional B cells are frequently elevated whereas mature naïve B cells and memory B cells are often decreased, along with defects in class-switch recombination.1,5,13 Hyperactive PI3Kδ signaling also drives terminal differentiation in B cells: immunoglobulin M+ (IgM+) plasma cells are often increased in patients with APDS.15,16 As a result of these disrupted lymphocyte subsets, many patients with APDS display elevated IgM and low IgG and IgA, with poor specific antibody responses, diminishing their ability to fight infections.5,6,13 The percentage of naïve B cells out of the total B cells reflects altered B-cell subsets and can provide a sense of the impact of APDS on immunodeficiency.
Lymphoproliferation, including lymphadenopathy, splenomegaly, hepatomegaly, and nodular lymphoid hyperplasia of the airways and gut is typical.5-8 Lymphadenopathy is driven by the proliferation of B cells and the infiltration of T follicular helper cells that are highly positive for programmed cell death protein 1 (PD-1).5,6 T-cell subsets skewed towards the effector phenotype may also contribute to lymphadenopathy because these cells demonstrate an enhanced proliferative burst on encounter with antigen.1 Depending on the location and size of the swollen lymph nodes, they may affect breathing or cause obstruction.17,18 Lymphadenopathy and expansion of lymphoid tissues primarily reflects the immune dysregulation aspect of APDS and its reduction, especially in the neck, mediastinum, and gut, improves organ specific function.
Other manifestations of APDS include autoimmunity (eg, cytopenias) and enteropathy.5-8 Uncontrolled antigen- or EBV-driven PI3Kδ activity may partially drive malignant transformation of B cells.19 Lymphoma has been reported in up to 25% of patients with APDS, at rates much higher than those of the general population.5-8,20 Less well-characterized manifestations include EBV positive posttransplant lymphoproliferative disease-like lymphoproliferation, sclerosing cholangitis, nodular regenerative hyperplasia of the liver with portal hypertension, allergy, asthma, eczema, neurodevelopmental delay, and seizures.5,6,8,21,22 End-organ damage, including hearing loss, bronchiectasis, and liver disease reflects the complex and progressive nature of APDS with multisystem involvement.5-8,23 APDS may lead to early mortality, although disease severity and survival remain variable.8,13
Current APDS management is mainly empirical. Treatment burden includes immunomodulatory therapies, prophylactic antimicrobials, immunoglobulin replacement therapy (IRT), and procedures such as splenectomies, repeated otosinopulmonary surgeries, and hematopoietic stem cell transplantation. Most strategies do not target the disease pathogenesis, hyperactive PI3Kδ signaling.5-7
Leniolisib, a novel, orally bioavailable small molecule inhibitor, was engineered to selectively target PI3Kδ signaling.24 We previously reported leniolisib use in 6 patients with APDS in a 12-week dose-finding clinical trial. Leniolisib was well tolerated, reduced PI3Kδ pathway hyperactivity, partially reconstituted lymphocyte subsets, and decreased lymphoproliferation.25
Here we report outcomes from a phase 3 trial (NCT02435173), a 12-week, randomized, triple-blinded, placebo-controlled, fixed-dose study of 31 patients.
Methods
Patients
Male and female patients aged 12 to 75 years and weighing ≥45 kg with pathogenic variants in PIK3CD or PIK3R1, clinical findings consistent with APDS, and ≥1 measurable lymph node on computed tomography or magnetic resonance imaging scan were eligible. Patients taking immunosuppressive agents, including rapamycin, underwent washout periods before entry. Glucocorticoid doses equivalent to ≤25 mg per day of prednisone were allowed within 2 weeks before the first dosing and throughout the study. supplemental Section 1 lists other eligibility criteria.
Trial design and treatment
Patients from the United States, Europe, and Russia were enrolled in a 12-week, randomized, subject-, investigator-, and sponsor-blinded, placebo-controlled, fixed-dose study. On day (D) 1, patients were randomized 2:1 to receive 70 mg leniolisib or placebo orally every 12 hours twice daily. Efficacy and safety assessments were performed on D15, D29, D57, and D85, with pharmacokinetic (PK) assessments on D29, D57, and D85.
Endpoints and assessments
There were 2 coprimary endpoints. One was the negative change from baseline at D85 in the log10-transformed sum of product diameters of the index lymph nodes. Index nodes comprised ≤6 of the largest lymph nodes as measured by magnetic resonance imaging or computed tomography scan and were selected per Cheson criteria.26 The other was the positive change from baseline at D85 in the percentage of naïve B cells out of total B cells as assessed by flow cytometry. In order to score an improvement in the naïve B-cell percentages, patients were required to have <48% naïve B cells at baseline for analysis.27
Secondary and exploratory endpoints included changes in bidimensional size and 3D volume of the spleen/liver, immunophenotyping of B- and T-cell subsets, and levels of serum immunoglobulins, cytokines, chemokines, and inflammatory markers. EBV and CMV loads were measured. Outcomes reported by patient and clinicians were evaluated via multiple instruments. PK assessments were performed in all patients receiving leniolisib. Safety was assessed in all patients; supplemental Section 1 includes assessment tools and further details.
Trial oversight
Novartis AG, in collaboration with investigators, designed the study. Novartis AG oversaw its conduct, and Novartis AG and Pharming Group NV analyzed the data. The NCT02435173 trial was conducted and reported in accordance with the protocol (including the statistical analysis plan). Data were gathered locally, and protocol-defined laboratory samples and imaging were processed centrally. The trial was conducted in accordance with the Declaration of Helsinki and the International Council on Harmonization Good Clinical Practice guidelines. Independent ethics committees or institutional review boards at each center approved the protocol. Patients and/or their guardians provided written informed consent/assent. An independent data and safety monitoring committee monitored safety and protocol compliance.
Statistics
Analysis of the primary lymphadenopathy endpoint determined sample size. Based on the standard deviation (SD) observed in the dose-finding trial (0.14), a sample size of 20 leniolisib and 10 placebo patients was estimated to provide 97% power to detect statistically significant differences using 5% type I error.
Three data sets were analyzed. The safety analysis set included all patients who received any study drug. The pharmacodynamics (PD) analysis set included all patients who received any study drug with no protocol deviations and with relevant impact on endpoints. The PK analysis set included all patients with ≥1 valid PK concentration measurement who received any study drug with no protocol deviations and with relevant impact on PK data. For all data sets, there was no imputation of missing data.
Analysis of covariance was performed on the PD analysis set for each coprimary endpoint. Baseline intake of glucocorticoids and IRT were included as categorical (Yes/No) covariates. Comparison of the 2 treatment groups was 2-sided, with 5% type I error. Treatment was a fixed effect and baseline was a covariate. For the lymphadenopathy endpoint, patients with 0 nodes at D85 were excluded. For the B-cell endpoint, only patients with <48% naïve B cells at baseline were included in the primary analysis (B-PD data set). A supportive analysis using the full PD analysis set was also performed. As a sensitivity analysis, the change from baseline in naïve B-cell levels across other study dates was analyzed in the B-PD data set using a longitudinal mixed model, with treatment, time, treatment by time interaction, baseline, and baseline by time interaction as fixed effects. Multiplicity was controlled across the coprimary endpoints implicitly by requiring both to be statistically significant for a positive study.
Analyses of covariance with the same parameters as the primary endpoint analyses were also used to assess changes from baseline to D85 in the PD analysis set for patient-reported outcomes, non-index lymph nodes, and spleen/liver sizes. The same repeated measures analysis used for B-cell frequencies was used for patient-reported outcomes. For all secondary and exploratory endpoints, summary statistics were calculated; 95% confidence intervals (CIs) have not been adjusted for multiplicity and inferences drawn may not be reproducible. See supplemental Section 1 for details.
Results
Patients and treatments
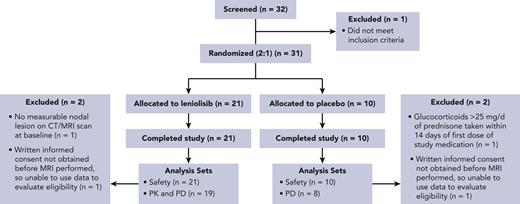
From December 2017 to August 2021, 32 patients were screened and 31 entered the trial (Figure 1). Baseline demographics and clinical characteristics of the patients were similar between groups and representative of the broader APDS population (Table 1, supplemental Section 2 and supplemental Table 1).7,8 All randomized patients completed treatment and were included in the safety analysis set. The PD analysis set (N = 27) was used to assess efficacy outcomes; 2 patients from each treatment arm were excluded because of protocol deviations. The B-PD analysis set for the primary naïve B-cell endpoint excluded an additional 14 patients; reasons included >48% naïve B cells at baseline (n = 8), no assessment performed at D85 (n = 4), no baseline measurement of total B-cell levels (n = 1), and no naïve B cells at baseline (n = 1). Baseline demographics and clinical characteristics of the B-PD analysis set were comparable between treatment arms (data not shown).
CONSORT participant flow diagram. CONSORT, Consolidated Standards of Reporting Trials; CT, computed tomography; MRI, magnetic resonance imaging.
CONSORT participant flow diagram. CONSORT, Consolidated Standards of Reporting Trials; CT, computed tomography; MRI, magnetic resonance imaging.
Baseline demographics and clinical characteristics (safety analysis set)
| . | Leniolisib (n = 21) . | Placebo (n = 10) . | Total (N = 31) . |
|---|---|---|---|
| Age, y | |||
| Median (range) | 20.0 (12-54) | 19.5 (15-48) | 20.0 (12-54) |
| <18, No. (%) | 8 (38.1) | 4 (40.0) | 12 (38.7) |
| Male/female sex, % | 52.4/47.6 | 40.0/60.0 | 48.4/51.6 |
| Weight, median (range), kg | 67.1 (46.9-100.6) | 68.9 (50.0-88.0) | 67.1 (46.9-100.6) |
| PIK3CD/PIK3R1 variant, No. | 16/5 | 9/1 | 25/6 |
| Baseline glucocorticoids,∗ No. (%) | 12 (57.1) | 6 (60.0) | 18 (58.1) |
| Baseline IRT, No. (%) | 14 (66.7) | 7 (70.0) | 21 (67.7) |
| Baseline antibiotic prophylaxis, No. (%) | 9 (42.9) | 4 (40.0) | 13 (41.9) |
| Previous sirolimus treatment, No. (%) | 4 (19.0) | 3 (30.0) | 7 (22.6) |
| Lymphoproliferation,† No. (%) | 15 (71.4) | 7 (70.0) | 22 (71.0) |
| Chronic infections, No. (%) | 18 (85.7) | 7 (70.0) | 25 (80.6) |
| Pulmonary disease, No. (%) | 14 (66.7) | 8 (80.0) | 22 (71.0) |
| Bronchiectasis | 8 (38.1) | 8 (80.0) | 16 (51.6) |
| Asthma | 7 (33.3) | 4 (40.0) | 11 (35.5) |
| Cytopenias, No. (%) | 13 (61.9) | 5 (50.0) | 18 (58.1) |
| Gastrointestinal disease, No. (%) | 10 (47.6) | 7 (70.0) | 17 (54.8) |
| . | Leniolisib (n = 21) . | Placebo (n = 10) . | Total (N = 31) . |
|---|---|---|---|
| Age, y | |||
| Median (range) | 20.0 (12-54) | 19.5 (15-48) | 20.0 (12-54) |
| <18, No. (%) | 8 (38.1) | 4 (40.0) | 12 (38.7) |
| Male/female sex, % | 52.4/47.6 | 40.0/60.0 | 48.4/51.6 |
| Weight, median (range), kg | 67.1 (46.9-100.6) | 68.9 (50.0-88.0) | 67.1 (46.9-100.6) |
| PIK3CD/PIK3R1 variant, No. | 16/5 | 9/1 | 25/6 |
| Baseline glucocorticoids,∗ No. (%) | 12 (57.1) | 6 (60.0) | 18 (58.1) |
| Baseline IRT, No. (%) | 14 (66.7) | 7 (70.0) | 21 (67.7) |
| Baseline antibiotic prophylaxis, No. (%) | 9 (42.9) | 4 (40.0) | 13 (41.9) |
| Previous sirolimus treatment, No. (%) | 4 (19.0) | 3 (30.0) | 7 (22.6) |
| Lymphoproliferation,† No. (%) | 15 (71.4) | 7 (70.0) | 22 (71.0) |
| Chronic infections, No. (%) | 18 (85.7) | 7 (70.0) | 25 (80.6) |
| Pulmonary disease, No. (%) | 14 (66.7) | 8 (80.0) | 22 (71.0) |
| Bronchiectasis | 8 (38.1) | 8 (80.0) | 16 (51.6) |
| Asthma | 7 (33.3) | 4 (40.0) | 11 (35.5) |
| Cytopenias, No. (%) | 13 (61.9) | 5 (50.0) | 18 (58.1) |
| Gastrointestinal disease, No. (%) | 10 (47.6) | 7 (70.0) | 17 (54.8) |
Systemic glucocorticoids in a dose equivalent to ≤25 mg per day of prednisone within 2 weeks before first dosing of study medication were allowed.
Although all patients were required to have lymphadenopathy for trial inclusion, documented clinical history of lymphoproliferation (eg, lymphadenopathy, splenomegaly, hepatomegaly) varied.
Lymphoproliferation outcomes
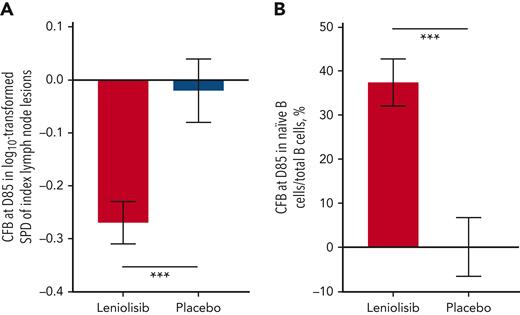
Leniolisib significantly reduced lymphadenopathy, meeting a coprimary endpoint (Figure 2A). The difference in the adjusted mean change (95% CI) between leniolisib (n = 18) and placebo (n = 8) was −0.25 (−0.38, −0.12; P = .0006). One patient receiving leniolisib was excluded from the PD analysis set because the baseline index node fully resolved by D85. Figure 3A shows index lymph node size for patients in the safety analysis set. In the safety analysis set, 26% of patients in the leniolisib group (n = 19) achieved complete absence of index lymphadenopathy, whereas the other 74% achieved partial response. In the placebo group (n = 9), 45% achieved partial response, 44% had stable disease, and 11% had an unknown response. The single patient with the unknown response moved during the scan, which may explain the data loss. Though no measurements were available at D85 for 2 of 6 index nodes, the rest demonstrated continued lymphadenopathy. Leniolisib decreased spleen size compared with placebo: the adjusted mean difference (95% CI) in bidimensional size between the groups was −13.5 cm2 (−24.1, −2.91; P = .0148) and in 3D volume was −186 cm3 (−297, −76.2; P = .0020). Of the patients in the safety analysis set with baseline splenomegaly, in the leniolisib group (n = 13), 38% achieved complete response, 54% achieved partial response, and 8% had stable disease at 12 weeks. In the placebo group (n = 5), 20% achieved complete response with the remaining 80% experiencing worsening disease. Figure 3B shows 3D spleen volumes of each patient with measurements in the safety analysis set. Figure 3C-J shows radiographic renderings of nodes and spleens. supplemental Section 2 reports supportive analyses and changes in liver size.
Coprimary endpoints. (A) Primary lymphadenopathy endpoint: least square mean of the change from baseline at D85 in the log10-transformed sum of product diameters of the index lymph node lesions in the PD analysis set (leniolisib, n = 18; placebo, n = 8). One patient in the leniolisib group was excluded because of complete resolution of lesions by D85 (0 mm), therefore the log10 transformation could not be computed. Compared with placebo, leniolisib reduced lymphadenopathy (P = .0006). (B) Primary naïve B-cell endpoint: least square mean of the change from baseline at D85 in the percentage of naïve B cells (CD19+CD27-CD10-) out of total B CD19+ cells in the B-PD analysis set (leniolisib, n = 8; placebo, n = 5). Compared with placebo, leniolisib increased naïve B-cell levels (P = .0002). Error bars are SEM. ∗∗∗P ≤ .001. CFB, change from baseline; SEM, standard error of mean.
Coprimary endpoints. (A) Primary lymphadenopathy endpoint: least square mean of the change from baseline at D85 in the log10-transformed sum of product diameters of the index lymph node lesions in the PD analysis set (leniolisib, n = 18; placebo, n = 8). One patient in the leniolisib group was excluded because of complete resolution of lesions by D85 (0 mm), therefore the log10 transformation could not be computed. Compared with placebo, leniolisib reduced lymphadenopathy (P = .0006). (B) Primary naïve B-cell endpoint: least square mean of the change from baseline at D85 in the percentage of naïve B cells (CD19+CD27-CD10-) out of total B CD19+ cells in the B-PD analysis set (leniolisib, n = 8; placebo, n = 5). Compared with placebo, leniolisib increased naïve B-cell levels (P = .0002). Error bars are SEM. ∗∗∗P ≤ .001. CFB, change from baseline; SEM, standard error of mean.
Changes in lymphoproliferation parameters. (A) Individual untransformed SPD of index lymph nodes (leniolisib, n = 19; placebo, n = 9). Reference range (≤1.5 × 1.5 cm) not shown as up to 6 lymph nodes may be counted per patient.26 (B) Individual spleen volumes (leniolisib, n = 20; placebo, n = 9). The gray box indicates the reference range for adults.28 All patients from the safety analysis set with measurements are included in (A) and (B), whereas efficacy analyses used the PD analysis set. (C-J) Radiographic renderings of spleen volume (C, D, G, H) and lymph node diameters (E, F, I, J) at screening and D85 from 2 patients in the leniolisib arm. The images in the top row (C-F) are from a 29-year-old female. The investigator diagnosed splenomegaly at screen (C) that was deemed absent by D85 (D). The index axillary lymph node lesion outlined in red decreased in size over the trial (E-F). Yellow and purple outlines in (F) indicate non-index lymph nodes. The images in the bottom row (G-J) are from a 17-year-old male. The investigator diagnosed splenomegaly at screen (G) that remained present by D85 despite the spleen decreasing in size (H). The index upper cervical lymph node lesion outlined in red (I-J) decreased in size over the trial.
Changes in lymphoproliferation parameters. (A) Individual untransformed SPD of index lymph nodes (leniolisib, n = 19; placebo, n = 9). Reference range (≤1.5 × 1.5 cm) not shown as up to 6 lymph nodes may be counted per patient.26 (B) Individual spleen volumes (leniolisib, n = 20; placebo, n = 9). The gray box indicates the reference range for adults.28 All patients from the safety analysis set with measurements are included in (A) and (B), whereas efficacy analyses used the PD analysis set. (C-J) Radiographic renderings of spleen volume (C, D, G, H) and lymph node diameters (E, F, I, J) at screening and D85 from 2 patients in the leniolisib arm. The images in the top row (C-F) are from a 29-year-old female. The investigator diagnosed splenomegaly at screen (C) that was deemed absent by D85 (D). The index axillary lymph node lesion outlined in red decreased in size over the trial (E-F). Yellow and purple outlines in (F) indicate non-index lymph nodes. The images in the bottom row (G-J) are from a 17-year-old male. The investigator diagnosed splenomegaly at screen (G) that remained present by D85 despite the spleen decreasing in size (H). The index upper cervical lymph node lesion outlined in red (I-J) decreased in size over the trial.
Immune outcomes
As an indication of improvement in immunodeficiency, leniolisib significantly increased the percentage of naïve B cells, meeting the other coprimary endpoint (Figure 2B). The difference in the adjusted mean change (95% CI) between leniolisib (n = 8) and placebo (n = 5) from baseline to D85 was 37.30 (24.06, 50.54; P = .0002). The supportive analysis, which included all patients in the PD analysis set with measurements at baseline and D85, was consistent with the primary analysis: the adjusted mean difference (95% CI) between leniolisib (n = 13) and placebo (n = 8) was 27.94 (15.02, 40.85; P = .0003). A repeated measures sensitivity analysis confirmed these findings (supplemental Section 2, supplemental Figure 2). Figure 4A shows the mean percentage of naïve B cells over time for all patients in the safety analysis set with measurements.
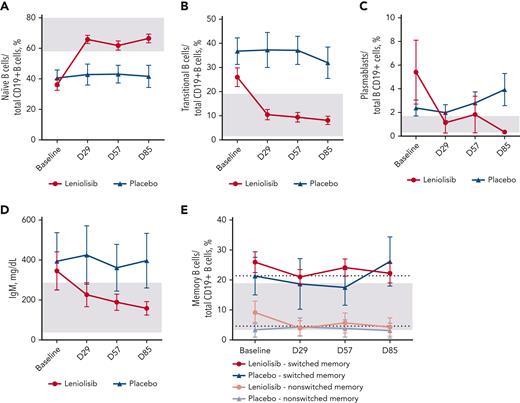
Changes in B-cell parameters. (A) Mean naïve B-cell percentages (CD19+CD27-CD10-) over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, 20, 19, 19, and 16; placebo, 10, 9, 9, and 10. (B) Mean transitional B-cell percentages (CD19+CD27-CD10+) over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, 19, 20, 20, and 17; placebo, 10, 8, 9, and 9. (C) Mean plasmablast percentage (CD19+CD27+CD38++) over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, 20, 20, 19, and 16; and placebo, 10, 9, 9, and 10. (D) Mean serum IgM level over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, 21, 20, 21, and 21; and placebo, 10, 10, 10, and 10. (E) Mean switched (CD19+CD27+IgD-) and nonswitched (CD19+CD27+IgD+) memory B-cell percentages over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, switched, 20, 19, 19, and 16; placebo, switched, 10, 8, 9, and 10; leniolisib, nonswitched, 20, 19, 20, and 17; and placebo, nonswitched, 10, 9, 9, and 10. Baseline was calculated as the average of D−1 and D1. All patients from the safety analysis set with measurements are included in the figures, whereas efficacy analysis of (A) used the B-PD analysis set. Gray boxes indicate reference range from literature (A-C,E) or laboratory (D).27,29 In (E), gray box indicates reference range for switched memory B cells and dotted line indicates reference range for nonswitched memory B cells. Error bars are SEM.
Changes in B-cell parameters. (A) Mean naïve B-cell percentages (CD19+CD27-CD10-) over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, 20, 19, 19, and 16; placebo, 10, 9, 9, and 10. (B) Mean transitional B-cell percentages (CD19+CD27-CD10+) over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, 19, 20, 20, and 17; placebo, 10, 8, 9, and 9. (C) Mean plasmablast percentage (CD19+CD27+CD38++) over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, 20, 20, 19, and 16; and placebo, 10, 9, 9, and 10. (D) Mean serum IgM level over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, 21, 20, 21, and 21; and placebo, 10, 10, 10, and 10. (E) Mean switched (CD19+CD27+IgD-) and nonswitched (CD19+CD27+IgD+) memory B-cell percentages over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, switched, 20, 19, 19, and 16; placebo, switched, 10, 8, 9, and 10; leniolisib, nonswitched, 20, 19, 20, and 17; and placebo, nonswitched, 10, 9, 9, and 10. Baseline was calculated as the average of D−1 and D1. All patients from the safety analysis set with measurements are included in the figures, whereas efficacy analysis of (A) used the B-PD analysis set. Gray boxes indicate reference range from literature (A-C,E) or laboratory (D).27,29 In (E), gray box indicates reference range for switched memory B cells and dotted line indicates reference range for nonswitched memory B cells. Error bars are SEM.
Leniolisib improved key lymphocyte subsets. Changes in frequency in the PD analysis set are shown in supplemental Table 3, whereas Figures 4-5 show all patients in the safety analysis set with measurements. Each stage of lymphocyte trafficking and development is impacted in APDS, as evidenced by the subsets of B cells that accumulate. Elevated transitional B cells and CD38+ plasmablasts decreased in the leniolisib group (Figure 4B-C). Switched and nonswitched memory B-cell percentages decreased slightly (Figure 4E). Importantly, leniolisib greatly reduced serum IgM, which is often elevated in patients with APDS (Figure 4D). In the PD analysis set, the mean IgM level decreased 208.26 mg/dL from baseline to D85 in the leniolisib arm and 10.00 mg/dL in the placebo arm.
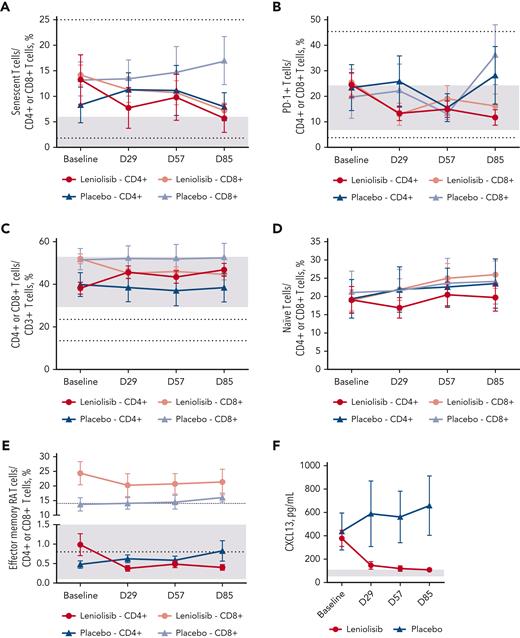
Changes in T-cell parameters. (A) Mean CD4+ and CD8+ senescent T-cell percentages (CD57+) over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, CD4+, 18, 15, 16, and 15; placebo, CD4+, 9, 9, 7, and 8; leniolisib, CD8+, 19, 16, 19, and 16; and placebo, CD8+, 10, 9, 7, and 8. (B) Mean CD4+ and CD8+ PD-1+ T-cell percentages over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, CD4+, 19, 17, 20, and 16; placebo, CD4+, 10, 9, 7, and 8; leniolisib, CD8+, 17, 14, 16, and 16; and placebo, CD8+, 10, 9, 7, and 7. (C) Mean CD4+ and CD8+ T-cell percentages over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, CD4+, 19, 17, 20, and 16; placebo, CD4+, 10, 9, 7, and 8; leniolisib, CD8+, 19, 17, 20, and 16; and placebo, CD8+, 10, 9, 7, and 8. (D) Mean naïve (CD45RA+CD62L+) CD4+ and CD8+ T-cell percentages over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, CD4+, 18, 17, 20, and 16; placebo, CD4+, 10, 9, 8, and 7; leniolisib, CD8+, 19, 17, 19, and 17; and placebo, CD8+, 10, 9, 7, and 8. Note that reference ranges are greater than graph axis, CD4+, 49.3% to 72.0%; CD8+, 48.6% to 87.5%.27 (E) Mean CD4+ and CD8+ terminally differentiated effector memory T-cell percentages (CD45RA+CD62L-) over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, CD4+, 18, 17, 19, and 16; placebo, CD4+, 10, 9, 7, and 8; leniolisib, CD8+, 19, 17, 20, and 16; and placebo, CD8+, 10, 9, 7, and 8. (F) Mean serum CXCL13 level over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, 21, 20, 21, and 21; and placebo, 10, 10, 10, and 9. Baseline was calculated as the average of D−1 and 1. All patients from the safety analysis set with measurements are included in the figures. Reference ranges are from personal communication (A-B; Manish Butte, University of California, Los Angeles, written communication, 29 March 2022), the literature (C-E), or laboratory (F).27 In (A-E), gray boxes indicate reference ranges for CD4+ cells, and dotted lines indicate reference ranges for CD8+ cells. In (F), gray box indicates reference range. Error bars are SEM.
Changes in T-cell parameters. (A) Mean CD4+ and CD8+ senescent T-cell percentages (CD57+) over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, CD4+, 18, 15, 16, and 15; placebo, CD4+, 9, 9, 7, and 8; leniolisib, CD8+, 19, 16, 19, and 16; and placebo, CD8+, 10, 9, 7, and 8. (B) Mean CD4+ and CD8+ PD-1+ T-cell percentages over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, CD4+, 19, 17, 20, and 16; placebo, CD4+, 10, 9, 7, and 8; leniolisib, CD8+, 17, 14, 16, and 16; and placebo, CD8+, 10, 9, 7, and 7. (C) Mean CD4+ and CD8+ T-cell percentages over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, CD4+, 19, 17, 20, and 16; placebo, CD4+, 10, 9, 7, and 8; leniolisib, CD8+, 19, 17, 20, and 16; and placebo, CD8+, 10, 9, 7, and 8. (D) Mean naïve (CD45RA+CD62L+) CD4+ and CD8+ T-cell percentages over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, CD4+, 18, 17, 20, and 16; placebo, CD4+, 10, 9, 8, and 7; leniolisib, CD8+, 19, 17, 19, and 17; and placebo, CD8+, 10, 9, 7, and 8. Note that reference ranges are greater than graph axis, CD4+, 49.3% to 72.0%; CD8+, 48.6% to 87.5%.27 (E) Mean CD4+ and CD8+ terminally differentiated effector memory T-cell percentages (CD45RA+CD62L-) over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, CD4+, 18, 17, 19, and 16; placebo, CD4+, 10, 9, 7, and 8; leniolisib, CD8+, 19, 17, 20, and 16; and placebo, CD8+, 10, 9, 7, and 8. (F) Mean serum CXCL13 level over time. n values for baseline, D29, D57, and D85 for each group are as follows: leniolisib, 21, 20, 21, and 21; and placebo, 10, 10, 10, and 9. Baseline was calculated as the average of D−1 and 1. All patients from the safety analysis set with measurements are included in the figures. Reference ranges are from personal communication (A-B; Manish Butte, University of California, Los Angeles, written communication, 29 March 2022), the literature (C-E), or laboratory (F).27 In (A-E), gray boxes indicate reference ranges for CD4+ cells, and dotted lines indicate reference ranges for CD8+ cells. In (F), gray box indicates reference range. Error bars are SEM.
CD8+ senescent CD57+ T cells and PD-1+ T cells, often elevated in patients with APDS, were reduced with leniolisib (Figures 5A-B). The inverted CD4:CD8 T-cell ratio normalized with leniolisib, increasing from 0.73 to 1.05. Although leniolisib treatment decreased the overall proportion of total CD8+ T cells, including terminally differentiated effector memory (TEMRA) CD8+ T cells, it increased naïve CD8+ T-cell percentages (Figure 5D-E). Leniolisib increased the percentage of total CD4+ T cells, with a decrease in the CD4+ TEMRA subset (Figure 5D-E). We observed little effect of leniolisib on central memory T cells and effector memory T cells after 12 weeks (supplemental Figure 2).
CXCL13 is a chemokine facilitating recruitment of B and T cells to lymphoid follicles and a marker for T follicular helper cell activity and aberrant lymphocyte trafficking; mean levels decreased 286.77 pg/mL from baseline to D85 in the leniolisib group and increased 59.31 pg/mL in the placebo arm in the PD analysis set (Figure 5F). We observed decreases in tumor necrosis factor α and other systemic inflammatory markers (supplemental Section 2, supplemental Figure 3).
Other secondary and exploratory outcomes
We used quantitative polymerase chain reaction to detect EBV and CMV. Although we detected CMV (n = 6), EBV (n = 5), or both (n = 5), no clinical progression occurred.30,31 The highest value for either virus occurred in 2 different patients: EBV, 696 DNA copies per mL; CMV, 591 DNA copies per mL.
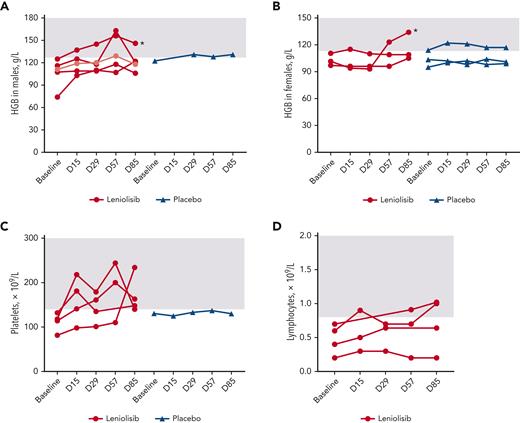
Baseline cytopenias varied among patients: in the leniolisib group, baseline cytopenias included anemia (n = 8), thrombocytopenia (n = 4), lymphopenia (n = 4), and neutropenia (n = 1), with 2 patients having multiple cytopenias. In the placebo group, baseline cytopenias included anemia (n = 4) and thrombocytopenia (n = 1). Among the safety analysis set, 82% of cytopenias improved in patients receiving leniolisib, compared with 60% in patients receiving placebo (supplemental Section 2; Figure 6).
Changes in baseline cytopenias. (A) Hemoglobin levels over time for individual male patients. Light red indicates patient whose lower limit of normal was 113 g/L, which was exceeded by D85. (B) Hemoglobin levels over time for individual female patients. (C) Platelet levels over time for individual patients. (D) Lymphocyte levels over time for individual patients. No patients in the placebo group had baseline lymphopenia. Baseline was calculated as the average of D−1 and D1. All patients from the safety analysis set with measurements are included in the figures. Gray boxes indicate reference range. In (A-B), asterisks indicate patients receiving iron. HGB, hemoglobin.
Changes in baseline cytopenias. (A) Hemoglobin levels over time for individual male patients. Light red indicates patient whose lower limit of normal was 113 g/L, which was exceeded by D85. (B) Hemoglobin levels over time for individual female patients. (C) Platelet levels over time for individual patients. (D) Lymphocyte levels over time for individual patients. No patients in the placebo group had baseline lymphopenia. Baseline was calculated as the average of D−1 and D1. All patients from the safety analysis set with measurements are included in the figures. Gray boxes indicate reference range. In (A-B), asterisks indicate patients receiving iron. HGB, hemoglobin.
We observed no statistically significant changes in patient- and clinician-reported outcomes in 12 weeks (supplemental Section 2 and supplemental Figures 4-6). However, patient and physician general assessment scores reflected clinically meaningful improvements for the leniolisib group, whereas the placebo group only showed meaningful improvement for the physician general assessment score. Elements of the Work Productivity and Activity Impairment plus Classroom Impairment Questionnaire also suggest clinically meaningful improvements for the leniolisib but not placebo group. Improvements in SF-36 scores were not clinically meaningful except for the physical functioning subscale for the placebo group. Corresponding investigator narratives described positive improvements including increased tolerance for physical activity and decreased fatigue in 70.0% of patients receiving leniolisib and 44.4% receiving placebo.
Pharmacokinetics
After a single dose of 70 mg leniolisib, the absorption phase was characterized by a geometric mean (CV %) Cmax of 2080 ng/mL (25.4%) reached at median Tmax of 2.87 hours (supplemental Figure 7A and supplemental Table 4). Multiple-dose PK was characterized by steady-state trough concentrations on D29, D57, and D85 (supplemental Figure 7B). The geometric mean (CV %) Ctrough on D85 was 804 ng/mL (67.5%).
Safety
Adverse events (AEs) were reported in 85.7% of patients receiving leniolisib and in 90.0% of the placebo group; these events were mostly grade 1 (74.2%; Table 2). Study drug–related AEs occurred in 8 patients; the incidence was lower in the leniolisib arm (23.8%) than in the placebo arm (30.0%). Transient alopecia was reported in 2 of 21 patients receiving leniolisib. In total, 5 patients reported a serious AE, with none judged as related to study medication. No deaths were reported within 30 days of the end of trial, and no AEs led to discontinuation of study treatment.
AEs through 30 days after end of trial
| . | Leniolisib (n = 21) . | Placebo (n = 10) . | Total (N = 31) . |
|---|---|---|---|
| Overall AE incidence∗,† | |||
| All, nE, nS (%) | 92, 18 (85.7) | 40, 9 (90.0) | 132, 27 (87.1) |
| Grade 1, nE, nS (%) | 65, 15 (71.4) | 26, 8 (80.0) | 91, 23 (74.2) |
| Grade 2, nE, nS (%) | 19, 9 (42.9) | 10, 5 (50.0) | 29, 14 (45.2) |
| Grade 3, nE, nS (%) | 3, 2 (9.5) | 4, 3 (30.0) | 7, 5 (16.1) |
| Grade 4, nE, nS (%) | 3, 2 (9.5) | 0, 0 (0.0) | 3, 2 (6.5) |
| Grade 5, nE, nS (%) | 0, 0 (0.0) | 0, 0 (0.0) | 0, 0 (0) |
| Study drug–related, nE, nS (%) | 6, 5 (23.8) | 8, 3 (30.0) | 14, 8 (25.8) |
| Serious, nE, nS (%) | 5, 3 (14.3) | 3, 2 (20.0) | 8, 5 (16.1) |
| Incidence of AEs by preferred term‡ | |||
| Headache, nS (%) | 5 (23.8) | 2 (20.0) | 7 (22.6) |
| Nausea, nS (%) | 1 (4.8) | 3 (30.0) | 4 (12.9) |
| Sinusitis, nS (%) | 4 (19.0) | 0 (0.0) | 4 (12.9) |
| Upper respiratory tract infection, nS (%) | 2 (9.5) | 2 (20.0) | 4 (12.9) |
| Fatigue, nS (%) | 2 (9.5) | 1 (10.0) | 3 (9.7) |
| Abdominal discomfort, nS (%) | 1 (4.8) | 1 (10.0) | 2 (6.5) |
| Alopecia, nS (%) | 2 (9.5) | 0 (0.0) | 2 (6.5) |
| Asthenia, nS (%) | 1 (4.8) | 1 (10.0) | 2 (6.5) |
| Back pain, nS (%) | 2 (9.5) | 0 (0.0) | 2 (6.5) |
| Diarrhea, nS (%) | 2 (9.5) | 0 (0.0) | 2 (6.5) |
| Dizziness, nS (%) | 1 (4.8) | 1 (10.0) | 2 (6.5) |
| Eczema, nS (%) | 2 (9.5) | 0 (0.0) | 2 (6.5) |
| Neck pain, nS (%) | 2 (9.5) | 0 (0.0) | 2 (6.5) |
| Pyrexia, nS (%) | 2 (9.5) | 0 (0.0) | 2 (6.5) |
| Urinary tract infection, nS (%) | 1 (4.8) | 1 (10.0) | 2 (6.5) |
| Vomiting | 1 (4.8) | 1 (10.0) | 2 (6.5) |
| Weight increased | 1 (4.8) | 1 (10.0) | 2 (6.5) |
| Incidence of treatment-related AEs as reported by study investigators | |||
| Alopecia, nS (%) | 2 (9.5) | 0 (0.0) | 2 (6.5) |
| Abdominal pain, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| Aphthous ulcer, nS (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Dyspnea, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| Fatigue, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| Headache, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| Taste disorder, nS (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Vasculitis, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| Vertigo, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| Vomiting, nS (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Weight increased, nS (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Incidence of serious AEs by preferred term§ | |||
| Alcohol poisoning, nS (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Coma, nS (%)‖ | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Dyspnea, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| Failure to thrive, nS (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Lipase increased, nS (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Lymphadenopathy, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| Mastoiditis, nS (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Urinary tract infection, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| . | Leniolisib (n = 21) . | Placebo (n = 10) . | Total (N = 31) . |
|---|---|---|---|
| Overall AE incidence∗,† | |||
| All, nE, nS (%) | 92, 18 (85.7) | 40, 9 (90.0) | 132, 27 (87.1) |
| Grade 1, nE, nS (%) | 65, 15 (71.4) | 26, 8 (80.0) | 91, 23 (74.2) |
| Grade 2, nE, nS (%) | 19, 9 (42.9) | 10, 5 (50.0) | 29, 14 (45.2) |
| Grade 3, nE, nS (%) | 3, 2 (9.5) | 4, 3 (30.0) | 7, 5 (16.1) |
| Grade 4, nE, nS (%) | 3, 2 (9.5) | 0, 0 (0.0) | 3, 2 (6.5) |
| Grade 5, nE, nS (%) | 0, 0 (0.0) | 0, 0 (0.0) | 0, 0 (0) |
| Study drug–related, nE, nS (%) | 6, 5 (23.8) | 8, 3 (30.0) | 14, 8 (25.8) |
| Serious, nE, nS (%) | 5, 3 (14.3) | 3, 2 (20.0) | 8, 5 (16.1) |
| Incidence of AEs by preferred term‡ | |||
| Headache, nS (%) | 5 (23.8) | 2 (20.0) | 7 (22.6) |
| Nausea, nS (%) | 1 (4.8) | 3 (30.0) | 4 (12.9) |
| Sinusitis, nS (%) | 4 (19.0) | 0 (0.0) | 4 (12.9) |
| Upper respiratory tract infection, nS (%) | 2 (9.5) | 2 (20.0) | 4 (12.9) |
| Fatigue, nS (%) | 2 (9.5) | 1 (10.0) | 3 (9.7) |
| Abdominal discomfort, nS (%) | 1 (4.8) | 1 (10.0) | 2 (6.5) |
| Alopecia, nS (%) | 2 (9.5) | 0 (0.0) | 2 (6.5) |
| Asthenia, nS (%) | 1 (4.8) | 1 (10.0) | 2 (6.5) |
| Back pain, nS (%) | 2 (9.5) | 0 (0.0) | 2 (6.5) |
| Diarrhea, nS (%) | 2 (9.5) | 0 (0.0) | 2 (6.5) |
| Dizziness, nS (%) | 1 (4.8) | 1 (10.0) | 2 (6.5) |
| Eczema, nS (%) | 2 (9.5) | 0 (0.0) | 2 (6.5) |
| Neck pain, nS (%) | 2 (9.5) | 0 (0.0) | 2 (6.5) |
| Pyrexia, nS (%) | 2 (9.5) | 0 (0.0) | 2 (6.5) |
| Urinary tract infection, nS (%) | 1 (4.8) | 1 (10.0) | 2 (6.5) |
| Vomiting | 1 (4.8) | 1 (10.0) | 2 (6.5) |
| Weight increased | 1 (4.8) | 1 (10.0) | 2 (6.5) |
| Incidence of treatment-related AEs as reported by study investigators | |||
| Alopecia, nS (%) | 2 (9.5) | 0 (0.0) | 2 (6.5) |
| Abdominal pain, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| Aphthous ulcer, nS (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Dyspnea, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| Fatigue, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| Headache, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| Taste disorder, nS (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Vasculitis, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| Vertigo, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| Vomiting, nS (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Weight increased, nS (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Incidence of serious AEs by preferred term§ | |||
| Alcohol poisoning, nS (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Coma, nS (%)‖ | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Dyspnea, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| Failure to thrive, nS (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Lipase increased, nS (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Lymphadenopathy, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
| Mastoiditis, nS (%) | 1 (4.8) | 0 (0.0) | 1 (3.2) |
| Urinary tract infection, nS (%) | 0 (0.0) | 1 (10.0) | 1 (3.2) |
nE, number of events in the category; nS, number of patients with at least 1 AE in the category; % is based on the number of patients.
Common Toxicity Criteria were used to determine AE grade. If grading did not exist for an AE, the following definitions were used: 1, mild; 2, moderate; 3, severe; 4, life-threatening; 5, death.
For AEs in ≥2 patients in total patients. A patient with multiple occurrences of an AE is counted only once in the AE category. Only AEs occurring at or after first drug intake are included.
A patient with multiple serious AEs with the same preferred term is counted only once for that preferred term.
The coma was a consequence of the alcohol poisoning.
Skin rash is a reported class effect of PI3Kδ inhibitors and was observed with leniolisib in healthy subjects at doses of 70 and 140 mg twice daily (<10% of subjects) as well as in patients with primary Sjögren syndrome at 70 mg twice daily (55% of patients).32,33 Here, 1 patient reported maculopapular rash (grade 1), which was not suspected to be related to leniolisib treatment. Immune-related AEs reported with the use of other PI3Kδ inhibitors for hematologic malignancies were not seen.32,34
Grade 3 or greater neutropenia is another class effect of PI3Kδ inhibitors seen in cancer treatment trials but was not seen in the present study.32,34 We observed transient neutropenia in the leniolisib group, with a nadir on D15 (mean count, 2.204 × 109/L) that recovered by D85. Four patients treated with leniolisib had neutrophil levels <1.0 × 109/L at a single visit, but none had infections, and at the subsequent visit counts were >1.0 × 109/L for all of them. Four of the 21 patients in the leniolisib arm developed asymptomatic mild neutropenia by D85 (0.90 × 109/L-1.30 × 109/L). Mean neutrophil level at D85 of all 21 patients receiving leniolisib was 2.94 × 109/L (range, 0.90 × 109/L-6.50 × 109/L). Mild and transient neutropenia was previously observed in healthy subjects treated with 70 mg leniolisib twice daily.
Discussion
In patients with APDS, leniolisib given orally at a dose of 70 mg twice daily over a period of 12 weeks reduced lymphadenopathy and increased naïve B-cell percentage significantly more than placebo, meeting both primary endpoints. Other key disease outcome measures, including spleen size, lymphocyte subsets, and cytopenias also improved. IgM levels decreased to healthy limits in most patients receiving leniolisib over 12 weeks. Changes in health-related quality of life measures were not statistically significant. However, investigator narratives reported a majority (70.0%) of patients receiving leniolisib (and 44.4% receiving placebo) had improvements in activity and energy levels.
We observed favorable changes in lymphocyte subsets attributed to improvements in trafficking, development, and maintenance of these cells. Mechanisms of defective EBV/CMV control in patients with APDS, such as elevated transitional B cells, which are thought to be entry points and reservoirs for EBV, and increased senescent CD8+ T cells, thought to be dysfunctional effectors, were all notably improved.13
Leniolisib was well tolerated. Many of the reported AEs were present at baseline. One patient in the leniolisib arm contracted asymptomatic COVID-19. Investigators reported more study drug–related AEs in the placebo group than the leniolisib group. Skin rash was reported in 1 patient receiving leniolisib but was judged to be fungal and not related to study treatment by the investigator, in contrast to effects reported with other PI3Kδ inhibitors used in non-APDS populations.32,33 This finding is consistent with that reported in part 1, in which we observed no skin rashes.25 We hypothesize that the AEs commonly associated with PI3Kδ inhibitors in other populations may be minimized in patients with APDS as their baseline hyperactive PI3Kδ is brought within a physiologic range and not inhibited completely by leniolisib.
This trial has several limitations. Firstly, the sample size was small, particularly for the naïve B-cell analysis, and immunophenotyping data were not available for all subsets for all patients. However, the supportive naïve B-cell analysis with the entire PD analysis set was consistent with results from the smaller data set. The sample size was decreased further because of protocol deviations: the analysis was based on treated patients with no relevant protocol deviations rather than intent-to-treat. The limited sample size might have also affected the randomization of disease complications and prior treatments; although most characteristics are similar between the 2 groups, bronchiectasis and gastrointestinal complications were more prevalent in the placebo group. Interestingly, the higher frequencies of these complications in the placebo group did not appear to correspond to disease impairment because the placebo group had less impairment on the Work Productivity and Activity Impairment plus Classroom Impairment Questionnaire than the leniolisib group (supplemental Figure 5). Regarding lung disease specifically, it is notable that patients with APDS who were well managed with adequate health care (eg, IRT) from early childhood did not develop significant bronchiectasis. Conversely, those patients who had clinically significant lung problems including surgical pulmonectomy and bronchiectasis did well on leniolisib and completed the 12-week clinical trial.
Secondly, the health-related quality of life surveys have not been validated in children or patients with inborn errors of immunity, which may contribute to nonsignificant results. Thirdly, conducting an international trial during a global pandemic entailed complications, including changes or limitations in performing assessments and procedures at predetermined time points. Finally, outcomes were evaluated over only a 12-week period, which was chosen to limit the amount of time that patients in the placebo group were without therapy. Washout periods were required for patients receiving antiproliferative, immunosuppressive, or B-cell depletion therapies before the trial and ranged from 6 weeks to 6 months, depending on the agent. Combined with the 12-week trial, patients in the placebo arm could have been without treatment for 4 to 8.5 months. Given the progressive nature of APDS, it would not have been ethical to withhold treatment from patients in the placebo group for longer. However, 12 weeks was insufficient to assess clinically meaningful measures, such as decreases in infection frequency, hospitalization, antimicrobial use, and continued need for IRT; these will be evaluated in the future. Pediatric trials in younger children with APDS are also planned.
Leniolisib is not the only PI3Kδ inhibitor that has been investigated for treatment of APDS. A phase 2 trial of the inhaled agent nemiralisib was completed in 5 patients but further studies were not pursued (NCT02593539).35 A phase 3 trial of the oral inhibitor seletalisib was terminated;36 published phase 1b and open label extension studies describe moderate efficacy and AEs that led to treatment discontinuation.37 The reasons behind the differences in efficacy and tolerability between seletalisib and leniolisib are not immediately clear but may involve inhibitor structure and isoform selectivity.
Overall, treatment with leniolisib demonstrated targeted therapy of a rare but biologically relevant immune dysregulation and immune deficiency with a significant benefit over placebo with respect to the coprimary endpoints, reducing lymphadenopathy and increasing the percentage of naïve B cells by D85, and was well tolerated in patients with APDS.
Acknowledgments
The authors thank the patients who took part in the study and their families. The authors also acknowledge the local physicians who cared for these patients: Jack H. Bleesing, Cincinnati Children’s Hospital Medical Center and University of Cincinnati College of Medicine, Cincinnati, OH; Zeynep Yesim Kucuk, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Jeffrey T. Trost, Stephen G. Diamantoni and Associates Family Practice, Quarryville, PA; Diana K. Baker, University of Iowa Carver College of Medicine, Iowa City, IA; Polly J. Ferguson, University of Iowa Carver College of Medicine, Iowa City, IA; Amy M. Scurlock, University of Arkansas for Medical Sciences and Arkansas Children’s Hospital, Little Rock, AR; Christa S. Zerbe, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD; Ivan Kingyue Chinn, Baylor College of Medicine and Texas Children’s Hospital, Houston, TX; Jill M. Powers, Grand River Medical Group, Dubuque, IA; Janna A. Kroiss, Aurora Family Medicine, Two Rivers, WI; John M. Routes, Medical College of Wisconsin, Milwaukee, WI; Yasmin W. Khan, Vanderbilt University Medical Center, Nashville, TN; Ramsay L. Fuleihan, Columbia University Irving Medical Center and New York-Presbyterian Morgan Stanley Children’s Hospital, New York, NY; Lee S. Clore, Jr, Allergy and Asthma Specialists, PSC, Owensboro, KY; Gary I. Kleiner, University of Miami Miller School of Medicine, Miami, FL; T. Prescott Atkinson, The University of Alabama at Birmingham, Birmingham, AL; Cecilia P. Mikita, Walter Reed National Military Medical Center, Bethesda, MD; Christopher V. Macomb, Walter Reed National Military Medical Center, Bethesda, MD; John Hansen-Flaschen, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA; Ganga R. Ranasuriya, University of Pittsburgh Medical Center Susquehanna Health, Lung Center, Williamsport, PA; Patricia A. Takach, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA; Maryam Ali Al-Nesf Al-Mansouri, Hamad Medical Corporation and Qatar University College of Medicine, Doha, Qatar; Saad Mujeb Al-Shareef, King Faisal Specialist Hospital, Riyadh, Saudi Arabia; Randa Arnaout, King Faisal Specialist Hospital, Riyadh, Saudi Arabia; and Clara Mosa, ARNAS Civico Di Cristina Benfratelli Hospital, Palermo, Italy. The authors thank Anuj Kashyap, Lavenda Chirombo Kluczynski, and Alanvin Orpia for their technical assistance, and Amy FitzPatrick, Carolyn Keating, and the staff at PRECISIONscientia for data collation and manuscript preparation.
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. The opinions expressed in this presentation are the authors’ own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the US government. Novartis Pharmaceuticals AG and Pharming Group NV provided funding for the clinical trials, and Pharming Healthcare, Inc, provided funding for the manuscript preparation by PRECISIONscientia.
Authorship
Contribution: V.K.R., S.W., A. Šedivá, A.P., C.S., A. Shcherbina, N.C., T.C., V.A.D., A.T., Y.Z., E.K., J.K., V.L., Y.R., and G.U. performed research and collected data; K.R. and K.K. were involved in the design of the research; J.B. and A.R. were involved in oversight of the data reports; and S.M.H. and M.J.L. maintained oversight of the NIH research.
Conflict-of-interest disclosure: A. Šedivá is a consult for Octapharma, Takeda, and Pharming Group NV. A. Shcherbina receives honoraria from and is a consult for Octapharma, CSL Behring, and Novartis AG. E.K. is an employee of Leidos Biomedical Research, Inc. V.A.D. is a consultant for and/or receives honoraria from AstraZeneca, Kedrion, Takeda, CSL Behring, Pfizer, and Pharming Group NV. V.A.D. also receives honoraria from Pfizer, and research funding from Takeda. K.K. is an employee and shareholder of Novartis Pharma AG. K.R. is an employee of Novartis Pharma AG. A.R. and J.B. are current employees and stock option holders of Pharming Group NV, and J.B. holds individual stock in NeoClone. The remaining authors declare no competing financial interests.
Correspondence: V. Koneti Rao, Room 10/12C106, 10 Center Drive, Bethesda, MD 20892; e-mail: .
References
Author notes
Pharming Group NV is committed to responsibly share data generated by interventional clinical trials with researchers. Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices) may be shared, including the study protocol and statistical analysis plan. Data will be made available beginning 12 months and ending 36 months following publication of this article. Researchers who have a methodologically sound research proposal should send inquiries or requests to info@pharming.com. Data requestors may be required to sign a data access agreement.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal