In this issue of Blood, Barilà et al1 characterize the clinical and biological features of Tγδ large granular lymphocyte leukemia (Tγδ LGLL). They report that Tγδ LGLL, compared with the more common Tαβ variant, displays distinctive features, is associated with a less indolent form of the disease, and has shorter overall survival (OS). Patients with Tγδ LGLL seem to benefit from therapy with noncytotoxic ciclosporin (CSA).
LGLL is a disease that is incompletely understood. The updated 2022 World Health Organization classification distinguishes 3 subtypes of monoclonal diseases of large granular lymphocytes (LGL): T cell–derived T-LGLL, the rarer natural killer (NK) cell–LGLL (both deemed rather indolent), as well as aggressive NK cell leukemia.
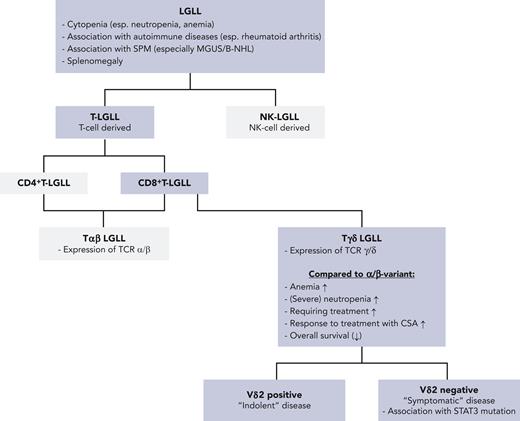
T-LGLL is commonly classified as a leukemia characterized by monoclonal cytotoxic T cells; however, it is a matter of debate whether this designation is adequate, as T-LGLL is not only a leukemia, but rather a disease characterized by (autoimmune-mediated) cytopenia, associated autoimmune disorders, and a disproportionate increase in secondary primary neoplasms, in particular, B-cell diseases. This triad is used to determine the indication for therapy.2
Phenotypically and clinically, a rarer variety, CD4+ T-LGLL, can be distinguished from CD8+ T-LGLL. Furthermore, within CD8+ T-LGLL a Tαβ variant can be distinguished from a Tγδ variant based on the T-cell receptor (TCR) chains expressed (see figure).
Dissecting LGLL. B-NHL, B-non-Hodgkin lymphoma; esp., especially; MGUS, monoclonal gammopathy of undetermined significance; SPM, second primary malignancy; STAT, signal transducer and activator of transcription.
Dissecting LGLL. B-NHL, B-non-Hodgkin lymphoma; esp., especially; MGUS, monoclonal gammopathy of undetermined significance; SPM, second primary malignancy; STAT, signal transducer and activator of transcription.
However, clonal LGLs do not necessarily indicate a disease; they are regularly detected after stem cell and organ transplantation. Furthermore, it is unclear how several borderline conditions such as Felty syndrome or hypoplastic myelodysplastic syndrome need to be classified within the LGLL landscape.
Barilà et al provide a further important piece to this puzzle: the largest cohort on Tγδ LGLL published to date with molecular characterization, as well as information on response to treatment. The authors collected data on 137 patients with Tγδ LGLL followed at 8 international centers and found that Tγδ LGLL is a variant with distinctive clinical features. Of special interest, Tγδ LGLL seemed significantly more frequently symptomatic with reduced OS compared with Tαβ LGLL.1 This finding contradicts the paradigm that both Tαβ LGLL and Tγδ LGLL are chronic conditions with a similar, rather indolent course of disease. However, Tγδ LGLL itself is not a homogenous entity: positivity for the Vδ2 receptor chain seems to be associated with a more indolent form of disease (see figure).
The analysis by Barilà et al further suggests that the choice of therapy also needs to be based on the subtype of T-LGLL: generally, independent of T-LGLL variant, low-dose methotrexate (MTX) or cyclophosphamide is used in first-line therapy, whereas immunosuppression with CSA is reserved for second- or third-line therapy.2 The data presented by Barilà et al suggest that “one treatment fits all” might no longer be correct for T-LGLL patients: patients with the γδ variant do not seem to respond well to MTX, but do benefit from therapy with CSA. The clinical relevance is emphasized by the observation that response to therapy translated into prolonged progression-free survival and prolonged OS.1
The differences found clinically between Tαβ and Tγδ LGLL by the authors are partially reflected by differences on the biological level. Under normal circumstances, Tγδ lymphocytes comprise less than 10% of peripheral blood CD3+ T cells. Unlike Tαβ lymphocytes, Tγδ lymphocytes are not dependent on the major histocompatibility complex for antigen presentation. Butyrophilin/butyrophilin-like proteins have been identified as potential specific antigen-presenting molecules in this cell subtype.3,4
The hypothesis that the initiating event of T-LGLL is chronic antigen stimulation is intriguing; however, tangible supporting evidence is missing so far. Indeed, in Tαβ LGLL TCR clonotypes have been demonstrated to be private to the disease (ie, absent in healthy controls) and to the patient,5,6 although a recent study observed that over half of the T-LGLL clonotype TCRs share structural similarities with TCR from the same patients’ nonleukemic repertoires.7 In Tγδ LGLL the dominant clonotypes seem to be mainly public (ie, shared with at least 1 healthy donor).8 At the genetic level, however, both subtypes appear to share somatic mutations as well as putative drivers.9
In interpreting the data presented by Barilà et al, some caution is still warranted:
Experience shows that the diagnosis of Tγδ LGLL is more difficult than that of Tαβ LGLL, partially due to the lower median LGL cell count. It is, therefore, possible that Tγδ LGLL cases are more likely to be late-stage diseases compared with Tαβ LGLL cases.
T-LGLL is also known to be associated with a variety of autoimmune cytopenia such as pure red cell aplasia (PRCA). Patients with PRCA respond well to noncytotoxic immunosuppression—for example, with CSA. Most patients that had received CSA in this cohort were treated due to anemia. Undiagnosed PRCA must be ruled out in this context, as Barilà et al mention in the Discussion. Provocatively, this raises the question whether careful diagnosis of the symptom determining concomitant disease is more important than differentiation of the T-cell receptor in choosing the optimal therapy for T-LGLL patients.
T-LGLL is a disease with a wide range of manifestations, and its position at the intersection of chronic inflammation, autoimmune disease, and monoclonal hematologic disease is highly intriguing.3 The major questions to be answered are:
What unites these clinical cases, and what separates them?
Is it one disease with a unifying pathophysiology or the reverse, clinically and/or molecularly distinct entities?
The exploration of the LGLL landscape has just begun. The heterogeneity of T-LGLL is not limited to the pathologic classification: even in a single patient, Tγδ, Tαβ, or NK LGLL clones can coexist. Furthermore, clonal drifts are common. The impact of genetic and epigenetic changes, not least on the interplay of the leukemic cells and the nonleukemic immune repertoire, is poorly understood. Here initial studies at the single-cell level have provided first exciting insights.7 These and future studies should pave the way for improved targeted and personalized therapies in this orphan disease.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal