Key Points
CH is associated with large-artery atherosclerosis, white matter lesion load, and a proinflammatory profile in patients with ischemic stroke.
CH and, in particular, mutations in TET2 and PPM1D are associated with higher risk for second vascular events and death after ischemic stroke.
Abstract
Clonal hematopoiesis (CH) is common among older people and is associated with an increased risk of atherosclerosis, inflammation, and shorter overall survival. Age and inflammation are major risk factors for ischemic stroke, yet the association of CH with risk of secondary vascular events and death is unknown. We investigated CH in peripheral blood DNA from 581 patients with first-ever ischemic stroke from the Prospective Cohort With Incident Stroke–Berlin study using error-corrected targeted sequencing. The primary composite end point (CEP) consisted of recurrent stroke, myocardial infarction, and all-cause mortality. A total of 348 somatic mutations with a variant allele frequency ≥1% were identified in 236 of 581 patients (41%). CH was associated with large-artery atherosclerosis stroke (P = .01) and white matter lesion (P < .001). CH-positive patients showed increased levels of proinflammatory cytokines, such as interleukin-6 (IL-6), interferon gamma, high-sensitivity C-reactive protein, and vascular cell adhesion molecule 1. CH-positive patients had a higher risk for the primary CEP (hazard ratio [HR], 1.55; 95% confidence interval [CI], 1.04-2.31; P = .03), which was more pronounced in patients with larger clones. CH clone size remained an independent risk factor (HR, 1.30; 95% CI, 1.04-1.62; P = .022) in multivariable Cox regression. Although our data show that, in particular, larger and TET2- or PPM1D-mutated clones are associated with increased risk of recurrent vascular events and death, this risk is partially mitigated by a common germline variant of the IL-6 receptor (IL-6R p.D358A). The CH mutation profile is accompanied by a proinflammatory profile, opening new avenues for preventive precision medicine approaches to resolve the self-perpetuating cycle of inflammation and clonal expansion.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 810.
Disclosures
Laurie Barclay, MD, freelance writer and reviewer, Medscape, LLC, has disclosed the following relevant financial relationships: stock, stock options, or bonds: AbbVie Inc. (former).
Learning objectives
Upon completion of this activity, participants will:
Determine the association of clonal hematopoiesis with large artery atherosclerosis, white matter lesion load, and proinflammatory profile in patients with ischemic stroke, based on an analysis of clonal hematopoiesis in peripheral blood DNA from 581 patients with first-ever ischemic stroke from PROSCIS-B
Evaluate clonal hematopoiesis clone dynamics and mutations associated with higher risk for second vascular events and death after ischemic stroke, based on an analysis of clonal hematopoiesis in peripheral blood DNA from 581 patients with first-ever ischemic stroke from PROSCIS-B
Assess clinical implications of the interplay of clonal hematopoiesis, systemic inflammation, and cardiovascular risk, based on an analysis of clonal hematopoiesis in peripheral blood DNA from 581 patients with first-ever ischemic stroke from PROSCIS-B
Release date February 16, 2023; Expiration date: February 16, 2024
Introduction
With aging, the risk of cardiovascular disease and cancer is increasing. Clonal hematopoiesis (CH), defined by the acquisition of somatic mutations in hematopoietic stem cells (HSCs), has been identified as a commonality between these 2 age-related conditions. CH occurs in 20% to 30% of individuals aged >60 years and most frequently involves mutations in epigenetic regulatory genes (eg, DNMT3A, TET2, and ASXL1).1-7 It is associated with a higher all-cause mortality, an increased risk for cardiovascular events, and an approximately 10-fold risk of developing hematologic malignancies.6,8 A causal relationship between CH and cardiovascular disease is best known for TET2, for which accelerated development of atherosclerosis driven by an altered function of the nucleotide-binding domain (NOD)-like receptor protein 3 (NLRP3)/interleukin-1β (IL-1β) inflammasome of mutated monocytes/macrophages was reported in preclinical models.8-10 Moreover, in patients with ischemic and nonischemic heart failure, CH has been reported to be associated with rapid progression and unfavorable overall survival.11,12 These data point toward multifaceted effects of mutated clones in CH-positive individuals, not only affecting self-renewal and differentiation but also inflammatory signaling of mature blood cells.13,14 Inflammation plays a crucial role in the pathogenesis of ischemic stroke and its functional consequences after brain injury.15-17 Compared with the rapidly increasing number of reports in the field of myocardial infarction (MI) and heart failure, little is known with respect to CH and ischemic stroke. In their original 2014 article, Jaiswal and colleagues reported an increased risk of ischemic stroke in individuals with CH, which has recently been confirmed in large patient series.6,18 To fill the knowledge gap concerning the role of CH in patients with ischemic stroke, we conducted a thorough genetic study investigating secondary cardiovascular risk of patients with ischemic stroke and CH in a large prospective cohort.19
Patients and methods
Patients
The Prospective Cohort With Incident Stroke–Berlin (PROSCIS-B; ClinicalTrials.gov identifier: NCT01363856) is a prospective, observational, hospital-based cohort study of patients enrolled after first-ever stroke. For further details, notice the previously published study protocol.19 In short, patients with ischemic stroke were recruited at 1 of 3 stroke units of Charité–Universitätsmedizin Berlin. Within 7 days of stroke onset, patients received interviews, extensive clinical examinations, and blood draws for laboratory analysis. During 3 years of follow-up, annual telephone-based interviews assessed patients’ vital status, incidence of cardiovascular diseases, and functional outcome. Vital status was additionally obtained from the local registry office, even if patients were lost to follow-up. Patients aged ≥18 years were included after first-ever stroke, as defined by World Health Organization criteria.20 Exclusion criteria were previous stroke (not counting transitory ischemic attacks), brain tumor or metastases, and participation in any intervention study. A Consolidated Standards of Reporting Trials (CONSORT) flow diagram for the inclusion and exclusion of patients is shown in supplemental Figure 1, available on the Blood website. In addition, serial blood samples of 24 patients with ischemic stroke from the Berlin Longterm Observation of Vascular Events Study (BeLOVE; German Clinical Trials Registry DRKS00016852) were investigated. BeLOVE is an ongoing observational clinical cohort study of patients with existing cardiovascular disease. The study was approved by the ethics committee (internal review board) of the Charité–Universitätsmedizin Berlin (EA1/218/09) and was conducted in accordance with ethical principles described in the Declaration of Helsinki.
Patient characteristics
Additional information at baseline was collected, including the following: sociodemographic parameters; etiologic subtype of stroke, according to the Trial of ORG 10172 in Acute Stroke Treatment classification21; stroke severity, according to National Institutes of Health Stroke Scale (NIHSS); cardiovascular risk factors (current smoking, alcohol consumption, hypertension, peripheral artery disease, prevalent atrial fibrillation, prevalent diabetes mellitus, and history of MI); and use of statin or antithrombotic/anticoagulant medication.
Outcome measures
Primary end point of the PROSCIS-B study is a composite end point (CEP) of recurrent stroke, MI, and all-cause death within 3 years, based on a structured interview with the patient or his/her relatives. Moreover, medical records were screened for any unreported end points. Reported recurrent stroke events or MIs were validated according to World Health Organization criteria22 using medical records from the responsible hospital and/or the treating physician by 2 senior vascular neurologists who were not involved in the PROSCIS-B study and were blinded to CH status. Survival status was obtained from the Berlin local registration office. Only confirmed end points were used in the analysis.
Targeted sequencing
Peripheral blood (PB) samples were obtained within 7 days after the incident stroke and stored at −80°C. DNA was extracted from PB samples and subjected to an error-corrected targeted sequencing workflow, as published previously.23,24 Briefly, sequencing libraries were prepared using a commercially available library preparation kit and a customized targeted sequencing panel (Twist BioScience) covering 45 genes recurrently mutated in CH (supplemental Table 1). Bioinformatic error correction was implemented via 9-bp unique molecular identifiers (xGen UDI-UMI adapters by Integrated DNA Technologies). Libraries were sequenced in paired-end mode on Illumina’s NovaSeq 6000 sequencing platform. Somatic variants with a variant allele frequency (VAF) ≥1% were identified using our in-house variant calling pipeline (see supplemental Patients and methods).24 Selected variants with VAF <2% were validated with an independent targeted ultradeep sequencing approach, as previously described.25,26 The genotype of the interleukin-6 receptor (IL-6R) single-nucleotide polymorphism p.D358A (rs2228145 C>A) was determined via Sanger sequencing (supplemental Patients and methods).
Biomarker analysis
Several inflammatory biomarkers (ie, high-sensitivity C-reactive protein [hsCRP], IL-1β, IL-6, IL-18, interferon gamma [IFN-γ], and tumor necrosis factor-α [TNF-α]) and markers of endothelial dysfunction (ie, vascular cell adhesion molecule 1 [VCAM-1] and soluble P-selectin) were measured in baseline serum samples at a commercial laboratory (Synevo, Clinical Trials Service Laboratory, Berlin, Germany). hsCRP was determined using the High Sensitivity C-Reactive Protein Assay (Siemens Healthcare, Germany) with a limit of detection of 0.3 mg/L and the IMMULITE 1000 system. IFN-γ, IL-6, IL-18, IL-1β, and TNF-α were determined using the MILLIPLEX Human Cytokine/Chemokine Magnetic Bead Panel multiplex assay (Merck, Germany) with a limit of detection of 3.20 pg/mL and the Luminex 200 System. VCAM-1 and soluble P-selectin were determined using the MILLIPLEX Human Cardiovascular Disease Magnetic Bead Panel 2 multiplex assay (Merck) with a limit of detection of 0.122 and 0.244 ng/mL, respectively, and the Luminex 200 System.
MRI data analysis
Magnetic resonance imaging (MRI) images were taken from clinical routine data using different 1.5- and 3-T scanners at 3 tertiary sites. Fluid-attenuated inversion recovery or T2-weighted images were used to assess white matter lesion severity, according to the Wahlund classification system.27 The Wahlund visual rating scale, or age-related white matter changes scale, is a scale ranging from a score of 0 to 30 that takes into account both sides of the brain (right and left) and prespecified brain regions (frontal, parieto-occipital, temporal, infratentorial/cerebellum, and basal ganglia). Herein, white matter hyperintensities are assigned a score of 0 to 3 in each region on both sides of the brain. The final score is the sum of all regions. Rating was performed independently by 2 raters (neurologist [A.K.] and senior neuroradiologist).
Statistical analysis
Statistical analysis was performed in R version 4.0.1 using the packages “ggplot2,” “stats,” “tableone,” “survival,” “powerSurvEpi,” and “forestmodel.” The primary end point was assessed using univariable and multivariable Cox regression models to obtain crude and adjusted hazard ratios (HRs). A sample size calculation was performed in advance using the “powerSurvEpi” package in R: Presuming a CH prevalence of 25%, a 2-tailed type I error α of 5%, and an HR of order 2.0 (based on the exploratory analysis for ischemic stroke and cardiovascular disease by Jaiswal et al6), 87 events were needed to obtain a power of 80%. Event-free survival curves were estimated using Kaplan-Meier analysis. To account for potential confounders, adjustment was made for baseline risk factors found to be imbalanced (P < .2 in Fisher test or Wilcoxon rank-sum test) between the groups of CH-positive and CH-negative individuals: age, sex, arterial hypertension, diabetes, obesity (defined as body mass index >30 kg/m2), smoking status, stroke severity (as measured by the NIHSS), peripheral artery disease, atrial fibrillation, physical activity before stroke incidence (low vs high), and use of anticoagulants. The Cox proportional hazards assumption was tested for all covariates using a score test of time-weighted residuals.28 The primary end point was assessed separately for the variables CH (all somatic mutations with VAF ≥1%), CH of indeterminate potential29 (CHIP; all somatic mutations with VAF ≥2%), CH with large clones (all somatic mutations with VAF ≥10%), as well as VAF as a continuous measure of clone size. In patients with >1 mutation, the largest VAF was used for clone size calculation. In analyses on single gene level, all patients carrying a mutation in the respective gene X were classified as X mutated, regardless of the VAF of the mutation or other comutations. Pairwise comparisons of variables for exploratory purposes were performed using Wilcoxon rank-sum tests or Fisher exact tests. The 2-sided level of significance was set at a P < .05 without adjustment for multiple testing, if not stated otherwise. In all analyses, we considered only complete cases.
Results
Sequencing data analysis
PB samples were available for 581 patients with incident ischemic stroke (supplemental Figure 1). We identified 348 somatic mutations with VAF ≥1% in 236 patients, representing 41% of our cohort. Although 155 patients harbored a single mutation, 81 patients harbored multiple (up to 5) mutations (Figure 1A; supplemental Figure 2). The VAF ranged from 1% to 48% (Figure 1B), with a median of 2.7%. ASXL1 mutations had a higher median VAF than non-ASXL1 mutations (5.6% vs 2.6%; P = .03; supplemental Figure 3). The most frequently mutated genes were DNMT3A, TET2, and ASXL1 (Figure 1C), affecting 81% of all CH-positive patients, consistent with the mutational spectrum described in other nonmalignant cohorts.6,26,30-32 The most frequent comutation pair was DNMT3A/TET2 in 19 patients (Figure 1D). TET2-mutated patients more frequently harbored a second mutation in another gene than DNMT3A-mutated patients (35/73 vs 37/119; P = .02). CBL-mutated patients frequently harbored second mutations (8/11), in particular DNMT3A mutations (Figure 1D; supplemental Figure 2).
Sequencing analysis and demographic and clinical characteristics of 581 patients with ischemic stroke from the PROSCIS-B study with respect to CH status. (A) Number of patients stratified by the number of detected mutations. (B) Histogram of VAF. (C) Gene-specific somatic mutation prevalence in 581 patients of the PROSCIS-B cohort. (D) CIRCOS plot (www.circos.ca) visualizing the comutational spectrum of the PROSCIS-B cohort. Segment length depicts number of patients with mutation(s) in the respective gene. Multiple mutations in the same gene are not considered. Ribbons depict the frequency of co-occurrence of 2 gene mutations in the same patient. (E) Age distribution of the PROSCIS-B cohort of CH-positive and CH-negative patients. (F) CH prevalence according to patient age. (G) CH prevalence according to etiological cause of stroke, as defined by the Trial of ORG 10172 in Acute Stroke Treatment criteria. Point size visualizes patient number, and color visualizes median age of the group.
Sequencing analysis and demographic and clinical characteristics of 581 patients with ischemic stroke from the PROSCIS-B study with respect to CH status. (A) Number of patients stratified by the number of detected mutations. (B) Histogram of VAF. (C) Gene-specific somatic mutation prevalence in 581 patients of the PROSCIS-B cohort. (D) CIRCOS plot (www.circos.ca) visualizing the comutational spectrum of the PROSCIS-B cohort. Segment length depicts number of patients with mutation(s) in the respective gene. Multiple mutations in the same gene are not considered. Ribbons depict the frequency of co-occurrence of 2 gene mutations in the same patient. (E) Age distribution of the PROSCIS-B cohort of CH-positive and CH-negative patients. (F) CH prevalence according to patient age. (G) CH prevalence according to etiological cause of stroke, as defined by the Trial of ORG 10172 in Acute Stroke Treatment criteria. Point size visualizes patient number, and color visualizes median age of the group.
Clonal dynamics after ischemic stroke
To investigate the dynamics of CH clones after ischemic stroke, we analyzed 24 CH-positive patients with ischemic stroke with available follow-up blood sample 2 years after stroke from the BeLOVE cohort. We identified 35 CH clones with VAF ≥1% in at least 1 time point. Median VAF increased from 1.7% to 2.2% (maximum increase, 3%; maximum decrease, 3%). To quantify clonal dynamics, we calculated the clonal fitness index following Robertson et al33 and classified clones as increasing, decreasing, or stable (supplemental Patients and methods). Median fitness was s = 0.09 per year, corresponding to a doubling time of ∼8 years for a clone of VAF 1%. Interestingly, we found that 13 of 35 clones showed positive selection and only 2 of 35 clones showed negative selection (supplemental Figure 4). On individual gene level, SRSF2-mutated clones showed the highest expansion properties (median fitness, s = 0.25 per year, corresponding to a doubling time of 2.8 years; supplemental Figure 5).
Associations of CH with baseline clinical characteristics
Baseline characteristics of the 581 patients by CH status are shown in Table 1. As previously reported, the presence of CH mutations correlated strongly with patient age, ranging from 0% in patients aged <40 years to >60% in patients aged >80 years (Figure 1E-F). In terms of the different etiological stroke subtypes according to the Trial of ORG 10172 in Acute Stroke Treatment criteria,21 the highest prevalence of CH was found in patients with evidence of large-artery atherosclerosis (49.7% vs 37.4% in patients with all other stroke subtypes; P = .01; Figure 1G). This association remained significant after correction for age, sex, and cardiovascular risk factors in a logistic regression (odds ratio, 1.67; 95% confidence interval [CI], 1.1-2.54; P = .016; supplemental Table 2). In terms of laboratory measures, CH-positive patients had lower baseline hemoglobin values (14.1 vs 14.4 g/dL; P = .04) and red blood cell counts (4.58/μL vs 4.72/μL; P = .007), as well as a lower estimated glomerular filtration rate (74.3 vs 83.3 ml/min per 1.73 m2; P < .001; supplemental Figure 6), as recently reported in patients with chronic kidney disease.34
Demographic and clinical characteristics of the PROSCIS-B cohort
| Characteristic . | CH negative (n = 345) . | CH positive (n = 236) . | Total (n = 581) . | P value∗ . |
|---|---|---|---|---|
| Age, median (IQR), y | 64 (55-73) | 73.5 (66.75-81) | 68 (59-76) | <.001 |
| Sex, no. (%) | .012 | |||
| Female | 227 (65.8) | 130 (55.1) | 357 (61.4) | |
| Male | 118 (34.2) | 106 (44.9) | 224 (38.6) | |
| TOAST classification, no. (%) | .037 | |||
| Large-artery atherosclerosis | 77 (22.3) | 76 (32.2) | 153 (26.3) | |
| Cardiac embolism | 80 (23.2) | 60 (25.4) | 140 (24.1) | |
| Small-artery occlusion | 61 (17.7) | 32 (13.6) | 93 (16.0) | |
| Other determined cause | 12 (3.5) | 4 (1.7) | 16 (2.8) | |
| Undetermined cause | 115 (33.3) | 64 (27.1) | 179 (30.8) | |
| Modified ranking scale, no. (%) | 1 | |||
| ≤1 | 224 (64.9) | 153 (64.8) | 377 (64.9) | |
| ≥2 | 121 (35.1) | 83 (35.2) | 204 (35.1) | |
| NIHSS score, no. (%) | .08 | |||
| ≤4 | 268 (77.7) | 168 (71.2) | 436 (75.0) | |
| >4 | 77 (22.3) | 68 (28.8) | 145 (25.0) | |
| Smoking status, no. (%) | .002 | |||
| Never smoker | 114 (33.4) | 108 (46.8) | 222 (38.8) | |
| Current or former smoker | 227 (66.5) | 123 (53.2) | 350 (61.2) | |
| Arterial hypertension, no. (%) | .01 | |||
| No | 136 (39.4) | 68 (28.8) | 204 (35.1) | |
| Yes | 209 (60.6) | 168 (71.2) | 377 (64.9) | |
| Diabetes mellitus, no. (%) | .066 | |||
| No | 279 (80.9) | 175 (74.2) | 454 (78.1) | |
| Yes | 66 (19.1) | 61 (25.8) | 127 (21.9) | |
| Dyslipidemia, no. (%) | .754 | |||
| No | 272 (79.8) | 183 (78.5) | 455 (79.3) | |
| Yes | 69 (20.2) | 50 (21.5) | 119 (20.7) | |
| Obesity, no. (%) | .022 | |||
| No | 250 (72.7) | 186 (80.9) | 436 (76.0) | |
| Yes | 94 (27.3) | 44 (19.1) | 138 (24.0) | |
| Atrial fibrillation, no. (%) | .01 | |||
| No | 283 (82.0) | 172 (72.9) | 455 (78.3) | |
| Yes | 62 (18.0) | 64 (27.1) | 126 (21.7) | |
| Coronary heart disease, no. (%) | .91 | |||
| No | 287 (83.2) | 198 (83.9) | 485 (83.5) | |
| Yes | 58 (16.8) | 38 (16.1) | 96 (16.5) | |
| Peripheral artery disease, no. (%) | .043 | |||
| No | 328 (95.1) | 214 (90.7) | 542 (93.3) | |
| Yes | 17 (4.9) | 22 (9.3) | 39 (6.7) | |
| History of cancer, no. (%) | .30 | |||
| No | 313 (92.3) | 210 (89.7) | 523 (91.3) | |
| Yes | 26 (7.7) | 24 (10.3) | 50 (8.7) | |
| Statin therapy, no. (%) | .41 | |||
| None | 50 (14.7) | 26 (11.1) | 76 (13.3) | |
| Low dose | 243 (71.7) | 178 (76.1) | 421 (73.5) | |
| High dose† | 46 (13.6) | 30 (12.8) | 76 (13.3) | |
| Antithrombotics, no. (%) | .19 | |||
| None | 0 (0.0) | 1 (0.4) | 1 (0.2) | |
| Antiplatelet | 271 (79.9) | 174 (74.4) | 445 (77.7) | |
| Anticoagulation | 61 (18.0) | 50 (21.4) | 111 (19.4) | |
| Both | 7 (2.1) | 9 (3.8) | 16 (2.8) | |
| IL-6R p.D358A, no. (%) | .069 | |||
| D/D | 148 (42.9) | 91 (38.6) | 239 (41.1) | |
| D/A | 146 (42.3) | 121 (51.3) | 267 (46.0) | |
| A/A | 51 (14.8) | 24 (10.2) | 75 (12.9) |
| Characteristic . | CH negative (n = 345) . | CH positive (n = 236) . | Total (n = 581) . | P value∗ . |
|---|---|---|---|---|
| Age, median (IQR), y | 64 (55-73) | 73.5 (66.75-81) | 68 (59-76) | <.001 |
| Sex, no. (%) | .012 | |||
| Female | 227 (65.8) | 130 (55.1) | 357 (61.4) | |
| Male | 118 (34.2) | 106 (44.9) | 224 (38.6) | |
| TOAST classification, no. (%) | .037 | |||
| Large-artery atherosclerosis | 77 (22.3) | 76 (32.2) | 153 (26.3) | |
| Cardiac embolism | 80 (23.2) | 60 (25.4) | 140 (24.1) | |
| Small-artery occlusion | 61 (17.7) | 32 (13.6) | 93 (16.0) | |
| Other determined cause | 12 (3.5) | 4 (1.7) | 16 (2.8) | |
| Undetermined cause | 115 (33.3) | 64 (27.1) | 179 (30.8) | |
| Modified ranking scale, no. (%) | 1 | |||
| ≤1 | 224 (64.9) | 153 (64.8) | 377 (64.9) | |
| ≥2 | 121 (35.1) | 83 (35.2) | 204 (35.1) | |
| NIHSS score, no. (%) | .08 | |||
| ≤4 | 268 (77.7) | 168 (71.2) | 436 (75.0) | |
| >4 | 77 (22.3) | 68 (28.8) | 145 (25.0) | |
| Smoking status, no. (%) | .002 | |||
| Never smoker | 114 (33.4) | 108 (46.8) | 222 (38.8) | |
| Current or former smoker | 227 (66.5) | 123 (53.2) | 350 (61.2) | |
| Arterial hypertension, no. (%) | .01 | |||
| No | 136 (39.4) | 68 (28.8) | 204 (35.1) | |
| Yes | 209 (60.6) | 168 (71.2) | 377 (64.9) | |
| Diabetes mellitus, no. (%) | .066 | |||
| No | 279 (80.9) | 175 (74.2) | 454 (78.1) | |
| Yes | 66 (19.1) | 61 (25.8) | 127 (21.9) | |
| Dyslipidemia, no. (%) | .754 | |||
| No | 272 (79.8) | 183 (78.5) | 455 (79.3) | |
| Yes | 69 (20.2) | 50 (21.5) | 119 (20.7) | |
| Obesity, no. (%) | .022 | |||
| No | 250 (72.7) | 186 (80.9) | 436 (76.0) | |
| Yes | 94 (27.3) | 44 (19.1) | 138 (24.0) | |
| Atrial fibrillation, no. (%) | .01 | |||
| No | 283 (82.0) | 172 (72.9) | 455 (78.3) | |
| Yes | 62 (18.0) | 64 (27.1) | 126 (21.7) | |
| Coronary heart disease, no. (%) | .91 | |||
| No | 287 (83.2) | 198 (83.9) | 485 (83.5) | |
| Yes | 58 (16.8) | 38 (16.1) | 96 (16.5) | |
| Peripheral artery disease, no. (%) | .043 | |||
| No | 328 (95.1) | 214 (90.7) | 542 (93.3) | |
| Yes | 17 (4.9) | 22 (9.3) | 39 (6.7) | |
| History of cancer, no. (%) | .30 | |||
| No | 313 (92.3) | 210 (89.7) | 523 (91.3) | |
| Yes | 26 (7.7) | 24 (10.3) | 50 (8.7) | |
| Statin therapy, no. (%) | .41 | |||
| None | 50 (14.7) | 26 (11.1) | 76 (13.3) | |
| Low dose | 243 (71.7) | 178 (76.1) | 421 (73.5) | |
| High dose† | 46 (13.6) | 30 (12.8) | 76 (13.3) | |
| Antithrombotics, no. (%) | .19 | |||
| None | 0 (0.0) | 1 (0.4) | 1 (0.2) | |
| Antiplatelet | 271 (79.9) | 174 (74.4) | 445 (77.7) | |
| Anticoagulation | 61 (18.0) | 50 (21.4) | 111 (19.4) | |
| Both | 7 (2.1) | 9 (3.8) | 16 (2.8) | |
| IL-6R p.D358A, no. (%) | .069 | |||
| D/D | 148 (42.9) | 91 (38.6) | 239 (41.1) | |
| D/A | 146 (42.3) | 121 (51.3) | 267 (46.0) | |
| A/A | 51 (14.8) | 24 (10.2) | 75 (12.9) |
Analyses were restricted to patients without missing values in the respective category.
IQR, interquartile range; TOAST, Trial of Org 10172 in Acute Stroke Treatment.
P value from Wilcoxon rank sum test or Fisher exact test.
High-dose statin therapy is defined as a dose equivalent to atorvastatin ≥ 40 mg/d.
Association of CH with inflammatory biomarkers
We measured levels of inflammatory biomarkers that have previously been related to CH (hsCRP, IL-1β, IL-6, TNF-α, IFN-γ, and IL-18) and markers of endothelial activation (VCAM-1 and P-selectin) at baseline in 562 patients. Patients with CH showed higher median values of hsCRP (6.1 [2.1-17.4] vs 4.0 [1.7-10.8] mg/mL; P = .006), IL-6 (4.9 [3.2-11.6] vs 3.7 [3.2-7.1] pg/mL; P = .002), and VCAM-1 (503 [412-605] vs 459.5 [370-571] ng/mL; P = .001) compared with patients without, at a Bonferroni-corrected significance level of P < .00625 (Figure 2A; supplemental Figure 7). The fraction of patients with elevated levels of IFN-γ was higher in patients with CH (21.7% vs 10.1%; P < .001; Figure 2A). In line with the previously reported association of TET2 deficiency with elevated IL-1β,8,9 we found higher IL-1β levels in patients with a TET2 mutation compared with CH-negative patients (3.20 [3.20-10.22] vs 3.20 [3.20-4.26] pg/mL; P = .015; Figure 2B).
Analysis of inflammatory biomarkers and white matter lesion load. (A) Boxplots of levels of inflammatory biomarkers at baseline of 562 patients with respect to CH mutation status as well as fraction of patients with IFN-γ values ≥ detection limit (3.20 pg/mL). (B) Boxplots of IL-1β levels in patients with a single TET2 mutation compared with CH-negative patients. (C) White matter lesion load in 398 patients with available MRI data, measured by the Wahlund score according to CH status. P values above brackets from Wilcoxon rank-sum test.
Analysis of inflammatory biomarkers and white matter lesion load. (A) Boxplots of levels of inflammatory biomarkers at baseline of 562 patients with respect to CH mutation status as well as fraction of patients with IFN-γ values ≥ detection limit (3.20 pg/mL). (B) Boxplots of IL-1β levels in patients with a single TET2 mutation compared with CH-negative patients. (C) White matter lesion load in 398 patients with available MRI data, measured by the Wahlund score according to CH status. P values above brackets from Wilcoxon rank-sum test.
Association of CH with white matter lesion load
White matter lesions (WMLs) are areas of abnormal myelination in the brain that reflect a mixture of inflammation, swelling, and damage to the myelin. There is increasing evidence that WMLs may be an early component of neurodegenerative conditions, such as Alzheimer disease and stroke.35 They can be detected in T2-weighted or fluid-attenuated inversion recovery sequences on MRI, are typically quantified by the Wahlund visual rating scale,27 and are considered a marker for small-vessel disease.35,36 Among 398 patients with available MRI data, the Wahlund score significantly differed between CH-positive and CH-negative patients (median, 7 [interquartile range, 4-11] vs 4 [interquartile range, 2-8]; P < .001; Figure 2C). As expected, WML load correlated with patient age (supplemental Figure 8A). However, we also found a statistically significant correlation of WML load with clone size (supplemental Figure 8B). In a linear regression with age, sex, arterial hypertension, diabetes mellitus, and clone size as independent variables, age (P < .0001), sex (P = .04), arterial hypertension (P = .007), and clone size (P = .06) were predictive for WML load (supplemental Figure 8C).
Associations of CH and clone size with recurrent vascular events and death
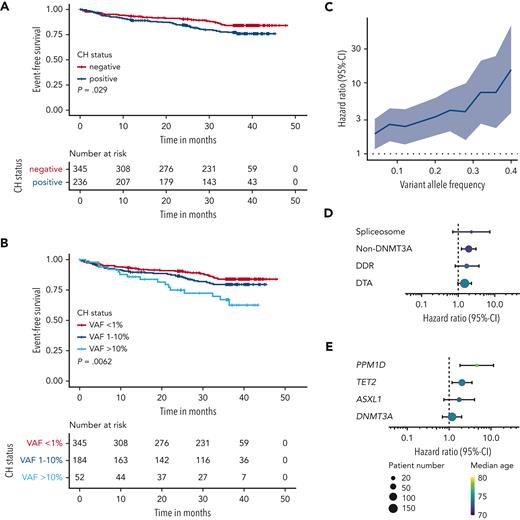
With a median follow-up time of 36.2 months, primary end point data were available for all 581 patients with a total of 97 events (50 deaths, 42 strokes, and 5 MIs). In a univariable time-to-event analysis, CH was associated with a higher risk of the occurrence of the CEP (HR, 1.55; 95% CI, 1.04-2.31; P = .03; Figure 3A). The risk was higher for patients with multiple mutations (HR, 1.94; 95% CI, 1.16-3.28; P = .01; supplemental Figure 9) and patients with large clone CH (defined by VAF ≥ 10%: HR, 2.45; 95% CI, 1.39-4.31; P = .002; Figure 3B; supplemental Figure 10). In fact, in a univariable Cox regression with the group of CH-negative patients as reference group, HR increases steadily with clone size (Figure 3C). This led us to investigate the effect of clone size as measured by the VAF as a continuous parameter. In a univariable Cox regression, clone size was associated with a 51% higher risk per 10% increase in VAF (HR, 1.51; 95% CI, 1.24-1.84; P < .001). Because of its strong association with CH, age represents a major source of confounding in our analyses. However, in a multivariable analysis with age, sex, NIHSS score, cardiovascular risk factors, atrial fibrillation, peripheral artery disease, and anticoagulant therapy as covariates, CH clone size remained an independent risk factor (HR, 1.30; 95% CI, 1.04-1.62; P = .022; Table 2). Of note, adding inflammatory biomarker levels associated with CH to the multivariable model did not substantially change the magnitude of this association (supplemental Table 3). On gene group level, in particular the group of non-DNMT3A (defined by the presence of a CH mutation[s] in other gene[s] than DNMT3A) was associated with higher risk of CEP occurrence in univariable analysis (Figure 3D), mainly driven by TET2 and PPM1D mutations (Figure 3E). In multivariable analysis, clone size of non-DNMT3A mutations (HR, 1.44 per 10% VAF; 95% CI, 1.16-1.79; P = .001), TET2 mutations (HR, 1.40 per 10% VAF; 95% CI, 1.01-1.91; P = .04), and PPM1D mutations (HR, 2.30 per 10% VAF; 95% CI, 1.37-3.87; P = .002) were significantly associated with the CEP (supplemental Table 4). Referring to the proposed definition of CH with indeterminate potential29 (CHIP), defined by CH with a VAF ≥2%, TET2-CHIP (HR, 2.25; 95% CI, 1.13-4.50; P = .021) and PPM1D-CHIP (HR, 6.75; 95% CI, 2.15-21.19; P = .001) were associated with a higher risk of CEP occurrence in multivariable analysis (supplemental Table 4).
Analysis of secondary vascular risk and mortality according to CH status. (A) Kaplan-Meier analysis of the CEP (recurrent stroke, myocardial infarction, and all-cause death) stratified by CH status. (B) Kaplan-Meier analysis of the CEP, stratified by CH status and clone size. (C) HR for the CEP from univariable Cox regression with varying VAF cutoff and CH-negative patients as reference group. The shaded area visualizes the 95% CI. (D and E) HRs for the CEP from univariable Cox regression for different gene groups (D) and different genes (E). Point size visualizes patient number, color visualizes median age of the group, and bars visualize the 95% CI. DDR, mutations in DNA damage response pathway genes (TP53, PPM1D, CHEK2, ATM, and RAD21); DTA, mutations affecting the genes DNMT3A, TET2, and ASXL1; non-DNMT3A, mutation(s) in gene(s) other than DNMT3A; spliceosome, SF3B1, SRSF2, and U2AF1.
Analysis of secondary vascular risk and mortality according to CH status. (A) Kaplan-Meier analysis of the CEP (recurrent stroke, myocardial infarction, and all-cause death) stratified by CH status. (B) Kaplan-Meier analysis of the CEP, stratified by CH status and clone size. (C) HR for the CEP from univariable Cox regression with varying VAF cutoff and CH-negative patients as reference group. The shaded area visualizes the 95% CI. (D and E) HRs for the CEP from univariable Cox regression for different gene groups (D) and different genes (E). Point size visualizes patient number, color visualizes median age of the group, and bars visualize the 95% CI. DDR, mutations in DNA damage response pathway genes (TP53, PPM1D, CHEK2, ATM, and RAD21); DTA, mutations affecting the genes DNMT3A, TET2, and ASXL1; non-DNMT3A, mutation(s) in gene(s) other than DNMT3A; spliceosome, SF3B1, SRSF2, and U2AF1.
Multivariable Cox regression model for the CEP
 |
 |
Multivariable Cox regression analysis of clone size (measured by the VAF) with potential confounders as covariates. For the variables VAF and age, HRs are given per 10% VAF change and per age decade, respectively.
To understand whether the risk for the CEP was concordant across vascular events and mortality, we separately analyzed for the end points recurrent stroke and death using Kaplan-Meier analysis. Interestingly, the increased risk for the CEP in CH-positive patients seems to be primarily driven by mortality rather than recurrent vascular events (supplemental Figure 11).
Interplay of CH, systemic inflammation, and IL-6R genotype
A common hypomorphic variant of the IL-6 receptor (IL-6R p.D358A; rs2228145 A>C) functionally impairs IL-6R signaling,37 is associated with reduced cardiovascular risk in large patient cohorts,38 and has been suggested as a proxy phenotype for IL-6 inhibition.39 Recently, Bick et al demonstrated that the risk attenuation attributable to the IL-6R p.D358A variant is more pronounced in the presence of CH.40 To explore the interplay of CH, systemic inflammation, and impaired IL-6R signaling in the context of ischemic stroke, we genotyped all 581 patients for the presence of the rs2228145 variant. The minor allele (C) frequency in our patient cohort was 35.9%. Although IL-6 levels did not significantly differ across different genotypes, individuals with A/D or A/A variants had significantly lower hsCRP values (Figure 4A-B). The IL-6R p.D358A variant was not associated with the CEP (HR, 0.93; 95% CI, 0.62-1.41; P = .76; supplemental Figure 12). When further stratifying by CH mutation status, however, our data indicate differences in the risk for CEP occurrence depending on CH/TET2 status (Figure 4C-F). The presence of A/D or A/A variants seems to partially attenuate the risk in the group of CH-positive and TET2-mutated patients, whereas it does not in CH/TET2-negative patients.
Interplay of clonal hematopoiesis, systemic inflammation, and IL-6R p.D358A. (A) IL-6 levels according to the IL-6R p.D358 variant. (B) hsCRP levels according to the IL-6R p.D358 variant. (C) Kaplan-Meier analysis of the CEP, stratified by CH status and IL-6R p.D358 variant. (D) Kaplan-Meier analysis of the CEP, stratified by TET2 mutation (mut.) status and IL-6R p.D358 variant. (E) HRs and 95% CI for the CEP from univariable Cox regression for CH-positive (pos.) patients, according to the IL-6R p.D358 variant compared with CH-negative (neg.) patients. (F) HRs and 95% CI for the CEP from univariable Cox regression for TET2-mutated patients, according to the IL-6R p.D358 variant compared with CH-negative patients. WT, wild type.
Interplay of clonal hematopoiesis, systemic inflammation, and IL-6R p.D358A. (A) IL-6 levels according to the IL-6R p.D358 variant. (B) hsCRP levels according to the IL-6R p.D358 variant. (C) Kaplan-Meier analysis of the CEP, stratified by CH status and IL-6R p.D358 variant. (D) Kaplan-Meier analysis of the CEP, stratified by TET2 mutation (mut.) status and IL-6R p.D358 variant. (E) HRs and 95% CI for the CEP from univariable Cox regression for CH-positive (pos.) patients, according to the IL-6R p.D358 variant compared with CH-negative (neg.) patients. (F) HRs and 95% CI for the CEP from univariable Cox regression for TET2-mutated patients, according to the IL-6R p.D358 variant compared with CH-negative patients. WT, wild type.
Discussion
In our large prospective cohort of patients with ischemic stroke, we found that CH is associated with a higher risk for recurrent vascular events and death. Associations with adverse outcome have likewise been documented for cardiovascular diseases, such as heart failure or aortic valve stenosis.41,42
Although the association of CH with secondary vascular risk is substantially driven by age, several arguments are in favor of an age-independent biologic effect of CH in this context. First, the risk for secondary vascular events in our cohort increased with clone size and number of mutations in the sense of a dose-response relation, remaining statistically significant after correction for age and other confounders. Clone size itself has been shown to be primarily a function of the mutation-specific clonal fitness rather than patient age (supplemental Figure 13).43,44 Similar effects of clone size have been reported in the risk prediction for hematologic malignancies.1,6,32
Second, we found statistically significant differences between the different mutated genes with respect to secondary vascular risk. Although mutations in the most frequently altered gene, DNMT3A, seem to confer a more benign phenotype, there were in contrast strong associations for mutations in genes other than DNMT3A, in particular TET2 and PPM1D, with recurrent vascular events and death. For these 2 genes, the proinflammatory dysregulation/pathway leading to accelerated development of atherosclerosis or heart failure is best understood.8-10,45
Third, we found stroke subtype-specific differences in the prevalence of CH. Although CH was in general highly prevalent in our cohort of patients with ischemic stroke, it was specifically associated with large-artery occlusion, supporting a potential role of CH in the pathogenesis of this stroke subtype. Compared with the gene mutation prevalence in a cohort of 237 patients with colorectal cancer of similar median age from the FIRE-3 study that was investigated with the exact same sequencing technique,24 we observed a significantly higher prevalence of TET2 mutations in patients with ischemic stroke (12.7% vs 5.9%; P = .004), whereas no significant difference was found for DNMT3A mutations (20.5% vs 18.6%; P = .56). This enrichment of TET2 mutations in patients with ischemic stroke further suggests a central role of TET2-driven CH in stroke pathogenesis.24
Recently, Bhattacharya et al demonstrated that CH is associated with higher stroke risk in individuals without prior stroke in large population-based cohorts.18 In contrast to our study, CH was associated with hemorrhagic stroke and small-vessel ischemic stroke in an exploratory analysis in one cohort sample of postmenopausal women.18 Although we cannot investigate the association with hemorrhagic stroke in the PROSCIS-B cohort, because recruitment resulted in predominantly patients with ischemic stroke being included, the reason for the differing findings concerning ischemic stroke subtypes presently remains unclear. Differences in study design (population based vs incident stroke) and sequencing sensitivity (whole exome sequencing vs error-corrected targeted sequencing) might, in part, explain diverging results. However, further studies, including mechanistic studies, will be necessary to determine cause-effect relations beyond the epidemiologic association level.
Following the current concept that CH-inducing mutations in HSCs increase the inflammatory properties of myeloid cells that differentiate from them,46 CH-positive patients showed elevated hsCRP and cytokine levels of IL-6 and IFN-γ at baseline after ischemic stroke. These findings hint toward a stronger systemic proinflammatory response as a reaction to ischemic brain damage. In addition, it is conceivable that resolution of acute inflammation in the brain after primary stroke might be affected in CH-positive patients as the monocyte/granulocyte axis represents both: a key regulator of inflammation resolution and a main target of mutated CH clones.47 Interestingly, our analysis of the IL-6R p.D358A single-nucleotide polymorphism indicates differences in cardiovascular risk modulation dependent on CH/TET2 status, as previously reported in large population studies without prevalent cardiovascular disease.40 These results warrant further studies in larger cohorts with potential implications for the applicability of anti-inflammatory treatment. Furthermore, we showed a previously unappreciated association between CH and elevated VCAM-1 levels. VCAM-1 mediates the adhesion of leukocytes to vascular endothelium and plays a critical role in the development of atherosclerosis.48 VCAM-1 is also an essential protein for homing and immune tolerance of HSCs.49
Although we comprehensively investigated CH in the by far largest cohort of patients with incident ischemic stroke to date, our study has several limitations. When dissecting the CEP into mortality and recurrent strokes, we find that the association is primarily driven by mortality. The cause of death is difficult to define in individuals after stroke because of significant comorbidities. Population-based studies suggest that approximately two-thirds of deaths are caused by cardiovascular events in patients after ischemic stroke.50 However, CH is also associated with a higher incidence of hematologic cancer. In the absence of a systematic documentation of cause of death, cancer incidence, or incidence of other vascular events in the PROSCIS-B study, it eventually remains elusive to which extent the reported association between CH and the CEP reflects secondary vascular risk. Moreover, our analyses rely on sequencing data of a single time point. As we exemplified in 24 CH-positive patients, assessing somatic mutations at 2 (or more) time points enables investigations of longitudinal clonal dynamics, which could provide additional insights into a potential relation between clonal fitness and clinical outcomes.
In summary, our results provide novel insights into the interplay of CH, systemic inflammation, and cardiovascular risk and set the stage for further investigations, potentially improving personalized risk stratification and secondary prevention strategies for patients experiencing ischemic stroke.
Acknowledgments
The authors thank all participating patients and their families of the Prospective Cohort With Incident Stroke–Berlin study cohort. The authors thank Tatiana Borodina and Jeannine Wilde for technical assistance and acknowledge the assistance of the Genomics Core Facility, Berlin Institute of Health/Max Delbrück Center and the assistance of the Central Biobank Charité (ZeBanC). This project was conducted with data from the Berlin Longterm Observation of Vascular Events Study (BeLOVE) cohort study. BeLOVE is substantially funded by the Berlin Institute of Health at Charité – Universitätsmedizin Berlin (BIH). We thank all participants who took part in BeLOVE and the staff in this research program.
This study was supported by grants from the Deutsche Krebshilfe (number 70113643) and the German Cancer Consortium, both awarded to F.D., and financially supported by the Berlin Center for Translational Vascular Biomedicine, a joint focus area by the BIH at Charité, Charité – Universitätsmedizin Berlin, and Max-Delbrück Center for Molecular Medicine. C.M.A. is a participant in the BIH Charité Junior Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin, and the Berlin Institute of Health at Charité (BIH). C.M.S. and S.D. were supported by a stipend from the Berlin School of Integrative Oncology. P.M.S. was supported by Postdoctoral Research Fellowship from Alexander von Humboldt Foundation. C.H. received funding from Deutsche Forschungsgemeinschaft (DFG) (HA5741/5-1, project numbers 417284923 and 424778381), Sonderforschungsbereich/Transregio 295, Bundesministerium für Bildung und Forschung (BMBF) Centrum für Schlaganfallforschung Berlin 01EO1301, and Fondation Leducq (ATTRACT 17 CVD 03). M.E. received funding from DFG under Germany’s Excellence Strategy (EXC-2049–390688087), BMBF, Deutsches Zentrum für Neurodegenerative Erkrankungen, Deutsches Zentrum für Herz-Kreislauf-Forschung, European Union, Corona Foundation, and Fondation Leducq. T.G.L. received funding from DFG.
Authorship
Contribution: C.M.A., T.G.L., M.E., and F.D. conceptualized the study; C.M.A., T.G.L., P.M.S., M.E., and F.D. performed methods; C.M.A. and R.H. performed software analysis; C.M.A., T.G.L., and R.H. performed formal analysis; C.M.A., T.G.L., P.M.S., A.K., P.L., S.H., C.M.S., S.K.P., M.T., P.S.S., S.D., P.U.H., C.H., M.E., and F.D. performed investigations; T.G.L., C.H., L.B., J.E.W., M.E., and F.D. obtained resources; C.M.A., P.M.S., and R.H. performed data curation; C.M.A. and F.D. performed writing (original draft preparation); C.M.A, P.M.S., T.G.L., M.E., and F.D. performed writing (review and editing); C.M.A. performed visualization; M.E. and F.D. supervised the study; M.E. and F.D. performed project administration; and M.E. and F.D. acquired funding; and all authors have read and agreed to the published version of the article.
Conflict-of-interest disclosure: F.D. reports personal fees from Gilead, Incyte, Roche, Novartis, AbbVie, and AstraZeneca, outside the submitted work. M.E. reports grants from Bayer and fees paid to the Charité from AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo, Amgen, GSK, Sanofi, Covidien, Novartis, and Pfizer, all outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Matthias Endres, Department of Neurology, Neurologie, Charité–Universitätsmedizin Berlin, Charitéplatz 1, 10117 Berlin, Germany; e-mail: matthias.endres@charite.de; and Frederik Damm, Department of Hematology, Oncology, and Cancer Immunology, Charité–Universitätsmedizin Berlin, Augustenburger Platz 1, 13353 Berlin, Germany; e-mail: frederik.damm@charite.de.
References
Author notes
∗C.M.A., T.G.L., and P.M.S. contributed equally to this work as first authors.
†M.E. and F.D. share senior authorship.
The data underlying this article will be shared on reasonable request to the corresponding authors.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal