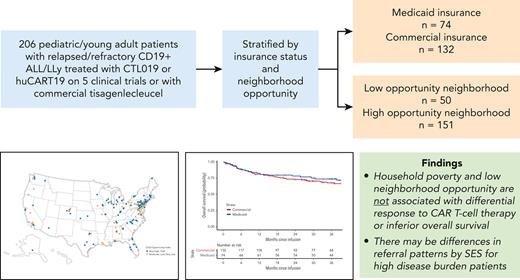
Key Points
CAR T-cell therapy produces equivalent survival for children with r/r ALL, regardless of household poverty or neighborhood opportunity.
CAR T-cell therapy may ameliorate factors influencing disparate outcomes observed in other treatment settings for children with ALL.
Abstract
Children living in poverty experience excessive relapse and death from newly diagnosed acute lymphoblastic leukemia (ALL). The influence of household poverty and neighborhood social determinants on outcomes from chimeric antigen receptor (CAR) T-cell therapy for relapsed/refractory (r/r) leukemia is poorly described. We identified patients with r/r CD19+ ALL/lymphoblastic lymphoma treated on CD19-directed CAR T-cell clinical trials or with commercial tisagenlecleucel from 2012 to 2020. Socioeconomic status (SES) was proxied at the household level, with poverty exposure defined as Medicaid-only insurance. Low-neighborhood opportunity was defined by the Childhood Opportunity Index. Among 206 patients aged 1 to 29, 35.9% were exposed to household poverty, and 24.9% had low-neighborhood opportunity. Patients unexposed to household poverty or low-opportunity neighborhoods were more likely to receive CAR T-cell therapy with a high disease burden (>25%), a disease characteristic associated with inferior outcomes, as compared with less advantaged patients (38% vs 30%; 37% vs 26%). Complete remission (CR) rate was 93%, with no significant differences by household poverty (P = .334) or neighborhood opportunity (P = .504). In multivariate analysis, patients from low-opportunity neighborhoods experienced an increased hazard of relapse as compared with others (P = .006; adjusted hazard ratio [HR], 2.3; 95% confidence interval [CI], 1.3-4.1). There was no difference in hazard of death (P = .545; adjusted HR, 1.2; 95% CI, 0.6-2.4). Among children who successfully receive CAR T-cell therapy, CR and overall survival are equitable regardless of proxied SES and neighborhood opportunity. Children from more advantaged households and neighborhoods receive CAR T-cell therapy with a higher disease burden. Investigation of multicenter outcomes and access disparities outside of clinical trial settings is warranted.
Introduction
Adverse social determinants of health contribute to significant disparities among children and adults in the United States.1,2 The field of oncology is no exception; among many of the most common pediatric and adult cancers, individuals living in poverty and those from historically marginalized racial and ethnic groups are significantly more likely to relapse and die from their disease than wealthier, White counterparts.3-7
Although survival outcomes have improved dramatically in recent decades in pediatric B-cell acute lymphoblastic leukemia (ALL), 20% of children relapse.8 Children from historically marginalized populations, including those living in poverty and those who identify as Black or Hispanic, are significantly more likely to both relapse and die from ALL, even when treated with highly standardized therapy on clinical trials.9 Furthermore, the difference in overall survival (OS) is even greater than event-free survival, suggesting that access to salvage therapy may contribute, in part, to differential outcomes.9
The question of differential access and outcome is highly salient in childhood B-ALL, given that nearly 1 in 5 children with cancer in the United States lives in poverty.10 Poverty-exposed children have higher rates of food insecurity,11 homelessness,12 psychological stress, and adverse childhood experiences,13 as well as presumed differential access to salvage therapy, all of which may potentially affect survival. Notably, neighborhood measures of adverse social determinants of health overlap with household-level social determinants of health in the United States but are distinct measurable constructs. In other high-resource therapies, such as hematopoietic stem cell transplantation (HSCT)14 and immunotherapy for neuroblastoma,15 neighborhood and household poverty have been associated with increased treatment-related mortality and inferior event-free and OS.
The emergence of precision medicine has led to many novel therapeutic options based on enhanced genomic signatures and molecular techniques,16 high resource and impactful interventions that raise significant concerns for worsening inequity within the American health care system based on differential access.17 Without close attention, precision medicine has the potential to simultaneously save the lives of some whereas making cure less attainable for others, thus worsening pre-existing inequities.18
Chimeric antigen receptor (CAR) T-cell therapy is highly efficacious for relapsed/refractory (r/r) B-ALL, with 1-year relapse-free survival (RFS) rates after CAR T-cell therapy approaching 60% to 80%.19-22 CAR T-cell therapy is a highly specialized treatment that requires clinical centers with formal accreditation,23 expertise, and resources for the collection, processing, administration, and toxicity management of cellular products. Given high resource use and center-specific limitations, this raises the question of whether CAR T-cell therapy is accessed equitably and if children from less resourced households experience equivalent outcomes with treatment. Despite these concerns, there are minimal data examining the influence of socioeconomic status on access to CAR T-cell therapy and outcomes with this salvage treatment. Understanding whether access and outcome disparities exist in the context of CAR T-cell therapy is essential to ensuring equity in the treatment of relapsed ALL.
To address this gap, we examined disease characteristics at the time of referral and survival outcomes in relation to proxied household poverty and neighborhood opportunity among children treated with CAR T-cell therapy for r/r ALL or lymphoblastic lymphoma (LLy).
Methods
Data source and study population
Children and young adults aged 1 to 29 years with r/r CD19+ ALL or LLy treated on 1 of 5 CD19-directed CAR T-cell clinical trials (clinicaltrials.govNCT01626495, NCT02435849, NCT02374333, NCT02228096, and NCT02906371)19,20,22,24 or with commercial tisagenlecleucel/Kymriah at The Children's Hospital of Philadelphia between April 2012 and December 2020 were included. To minimize heterogeneity, the cohort was restricted to patients who were CAR T-cell therapy–naïve (CONSORT diagram, supplemental Figure 1, available on the Blood website). Patients with an international primary address were excluded a priori owing to the inability to categorize proxied socioeconomic and neighborhood opportunity exposures. Data were derived from CAR T-cell clinical trial data sets and abstracted from the electronic medical record (EMR).
Patients or their parent/guardian provided written informed consent or assent, as appropriate, for treatment in each respective clinical trial. This retrospective analysis of trial and clinical data was approved by the CHOP Institutional Review Board.
Exposure: household poverty
Household poverty was the primary exposure of interest and proxied by patient insurance at the time of referral to CAR T-cell therapy. Insurance status was determined by manual review of the EMR and dichotomized as sole coverage by public insurance (Medicaid or Children’s Health Insurance Program) vs private or other (commercial, dual commercial and public as secondary insurer, military, other). Children with sole public insurance were considered household poverty–exposed.25,26
Exposure: neighborhood opportunity
Neighborhood opportunity was proxied by the 2015 Childhood Opportunity Index 2.0 (COI). The COI is a census tract–based multidimensional quality measure of US neighborhood metrics with an overall score and subscores across 3 domains of opportunity: education, health/environment, and social/economic.27 COI was computed based on EMR-abstracted home address at the time of CAR T-cell referral and geocoded to the census tract using ArcGIS Pro 2.5.1 (Esri, Redlands, CA) and the 2019 Esri Business Analyst composite address locator. Patient neighborhoods were dichotomized as low-neighborhood opportunity (quintiles 1-2) vs high-neighborhood opportunity (quintiles 3-5), with quintiles determined using national distribution.27 We additionally explored neighborhood opportunity using individual quintiles.
Outcomes
OS was the primary outcome defined as time from date of CAR T-cell infusion to date of death from any cause or censored at last contact. RFS was the secondary outcome defined as time from complete remission (CR; M1 bone marrow [<5% lymphoblasts] with trilineage hematopoiesis and no evidence of extramedullary disease) measured on first bone marrow evaluation 28 days after CAR T-cell infusion to the date of relapse or death, only among patients who achieved CR. For RFS, patients were censored for alternative cancer-directed therapy in remission (HSCT, chemotherapy, other immunotherapy or CAR T-cell therapy, tyrosine kinase inhibitors), subsequent malignancy, or at last contact, whichever event was earliest. CD19 status at relapse was characterized as positive or negative.
Covariates
Patient characteristics were abstracted from the EMR and included age at referral (<5, 5-9, 10-17, 18-29 years), sex (male, female), race, and ethnicity (non-Hispanic White, non-Hispanic Black or African American, Hispanic, or Other).28 Owing to small numbers, patients of self-reported American Indian (n = 3), Asian (n = 5), or unknown (n = 3) races were combined for statistical modeling as “Other.” A single patient identified as Hispanic-Black and was coded as Hispanic.
Tumor biology was abstracted from the EMR and trial databases. Risk stratification by cytogenetics was defined according to prior analysis.29 Clinical history at the time of referral included: disease status (primary refractory, first relapse, second relapse, third or greater relapse), prior therapies (blinatumomab, inotuzumab, HSCT, radiation), and baseline pre–CAR T-cell therapy disease burden (defined from the highest preinfusion evaluative bone marrow blast percentage; categorized as: <0.01, 0.01-4.99, 5-24.99 and ≥25). A majority of both commercial (96%) and trial (89%) patients underwent staging bone marrow aspirate and biopsy with multiparameter flow cytometry determination of minimal residual disease after lymphodepleting chemotherapy and before infusion; all others underwent staging before lymphodepleting chemotherapy.
Patients were categorized as local referral (Pennsylvania, New Jersey, and Delaware and previously followed at CHOP determined by manual chart review) or external referral (within catchment but not previously cared for at CHOP or outside of the 3-state catchment area) based on geographic location.
Statistical analysis
Follow-up data were current through 1 June 2021. Patient demographics, tumor, and treatment characteristics were summarized for the overall cohort by household poverty and neighborhood opportunity using descriptive statistics. Distributions of characteristics were compared using the Fisher exact test for categorical variables. OS was censored at the minimum median follow-up time across exposure groups in the primary analysis to ensure a comparable length of follow-up. Kaplan-Meier curves of OS and RFS were plotted by household poverty and neighborhood opportunity separately and compared using log-rank tests. Cox regressions were used to estimate unadjusted and adjusted hazard ratios (HR) of OS and RFS by household poverty status and neighborhood opportunity, with proportional hazard assumptions assessed by log-log plots.
Associations between poverty and neighborhood opportunity exposures, covariates, and survival outcomes were evaluated with univariate Cox proportional hazard models. Covariates associated with both poverty exposures and outcome (based on P < .2 or proportional difference of ≥10%) were included in the multivariate regression model. In addition to the primary analysis evaluating the overall COI score, secondary analyses also separately evaluated each of the 3 domains of COI.
Sensitivity analyses
We performed a series of sensitivity analyses to assess the robustness of our primary analyses of exposures and outcome. First, multivariate models excluding race and ethnicity as predictors were constructed to address potential overadjustment because of structural racism.30,31 Second, to control for potential dependent-censoring by covariates, additional covariates associated with censoring were adjusted for in the multivariate models even if they were not confounders. Third, time to relapse/death was analyzed, treating alternative cancer-directed therapy and subsequent malignancy as competing risks rather than censoring for RFS. Cumulative incidence functions for both the primary event (relapse/death) and competing risks were generated by binary exposure group and compared using the Gray test. Cause-specific hazard regression models for competing risks were used to assess the association between the exposures and the cause-specific hazard of relapse/death. Analyses were performed using Stata/BE version 17.0 and SAS 9.4 (SAS Institute, Cary, NC) statistical software.
Data sharing statement
Deidentified data will be shared with investigators who provide a methodologically sound proposal with appropriate Institution Review Board–approved aims, so long as release of those data does not compromise an ongoing trial or study.
Results
Characteristics of study population
The analytic cohort included 206 patients (median age 12.5 years, range 1.4-29.1) with relapsed or refractory CD19+ ALL or LLy treated at CHOP. Most patients received investigational CTL019/tisagenlecleucel (n = 128, 62.14%),32 45 (21.84%) received commercial Kymriah, and 33 (16.02%) received huCART19.24 The median length of follow-up was 46 months; 48 months for household poverty–unexposed vs 43 months for household poverty–exposed, and 48 months for high-neighborhood opportunity vs 39 months for low-neighborhood opportunity. Thus, all survival times were censored at 39 months.
Patients came from 38 US states and Puerto Rico (Figure 1). Five patients had addresses that could not be geocoded and were therefore excluded from the neighborhood-level analyses. Children exposed to household poverty were disproportionately Black or African American and Hispanic as compared with White non-Hispanic patients: 60% of Black patients, 70% of Hispanic patients, and 23% of non-Hispanic White patients were household poverty–exposed. Similar associations were observed for those living in low-neighborhood opportunity (Table 1). Notably, both household poverty–exposed (P = .010) and low-neighborhood opportunity–exposed (P = .049) patients were more likely to receive CAR T-cell therapy with lower marrow disease burden as compared with unexposed patients. Prior HSCT, blinatumomab exposure, cytogenetic risk category, and year of CAR T-cell infusion did not differ by household poverty and COI (Table 1). The proxied exposure groups identified distinct cohorts: 60% of low-neighborhood opportunity patients were household poverty–exposed, and 27% of high-neighborhood opportunity households were household poverty–exposed.
Baseline patient and disease characteristics
| . | Total (N = 206) . | Insurance status . | Neighborhood opportunity∗ . | ||||
|---|---|---|---|---|---|---|---|
| Private insurance (N = 132) . | Medicaid (N = 74) . | P . | High COI (N = 151) . | Low COI (N = 50) . | P . | ||
| Age categories, n (%) | .170 | .070 | |||||
| <5 y | 23 (11.2) | 15 (11.4) | 8 (10.8) | 19 (12.6) | 3 (6.0) | ||
| 5-9.99 y | 57 (27.7) | 30 (22.7) | 27 (36.5) | 35 (23.2) | 19 (38.0) | ||
| 10 to 17.99 y | 89 (43.2) | 63 (47.7) | 26 (35.1) | 65 (43.0) | 23 (46.0) | ||
| ≥18 | 37 (18.0) | 24 (18.2) | 13 (17.6) | 32 (21.2) | 5 (10.0) | ||
| Sex, n (%) | .770 | .620 | |||||
| Male | 120 (58.3) | 78 (59.1) | 42 (56.8) | 91 (60.3) | 28 (56.0) | ||
| Female | 86 (41.7) | 54 (40.9) | 32 (43.2) | 60 (39.7) | 22 (44.0) | ||
| Race and ethnicity, n (%) | <.001 | <.001 | |||||
| White non-Hispanic | 131 (63.6) | 101 (76.5) | 30 (40.5) | 109 (72.2) | 18 (36.0) | ||
| Black or African American | 15 (7.3) | 6 (4.5) | 9 (12.2) | 10 (6.6) | 5 (10.0) | ||
| Hispanic | 44 (21.4) | 13 (9.8) | 31 (41.9) | 20 (13.2) | 23 (46.0) | ||
| Other | 16 (7.8) | 12 (9.1) | 4 (5.4) | 12 (7.9) | 4 (8.0) | ||
| Cytogenetics category, n (%)† | .560 | .800 | |||||
| Favorable | 42 (20.4) | 29 (22.0) | 13 (17.6) | 34 (22.5) | 8 (16.0) | ||
| Intermediate risk | 24 (11.7) | 14 (10.6) | 10 (13.5) | 15 (9.9) | 7 (14.0) | ||
| High risk | 63 (30.6) | 37 (28.0) | 26 (35.1) | 45 (29.8) | 17 (34.0) | ||
| Uninformative | 71 (34.5) | 49 (37.1) | 22 (29.7) | 52 (34.4) | 17 (34.0) | ||
| Missing | 6 (2.9) | 3 (2.3) | 3 (4.1) | 5 (3.3) | 1 (2.0) | ||
| Disease status at referral, n (%) | .026 | .740 | |||||
| Primary refractory | 33 (16.0) | 28 (21.2) | 5 (6.8) | 25 (16.6) | 8 (16.0) | ||
| First relapse | 62 (30.1) | 35 (26.5) | 27 (36.5) | 47 (31.1) | 13 (26.0) | ||
| Second relapse | 77 (37.4) | 50 (37.9) | 27 (36.5) | 53 (35.1) | 22 (44.0) | ||
| Third or greater relapse | 34 (16.5) | 19 (14.4) | 15 (20.3) | 26 (17.2) | 7 (14.0) | ||
| Marrow status preinfusion, n (%) | .010 | .049 | |||||
| <0.01% | 76 (36.9) | 38 (28.8) | 38 (51.4) | 49 (32.5) | 26 (52.0) | ||
| 0.01% to 4.99% | 42 (20.4) | 33 (25.0) | 9 (12.2) | 35 (23.2) | 6 (12.0) | ||
| 5% to 24.99% | 16 (7.8) | 11 (8.3) | 5 (6.8) | 11 (7.3) | 5 (10.0) | ||
| >25% | 72 (35.0) | 50 (37.9) | 22 (29.7) | 56 (37.1) | 13 (26.0) | ||
| Previous HSCT | 88 (42.7%) | 53 (40.2%) | 35 (47.3%) | .380 | 63 (41.7%) | 23 (46.0%) | .620 |
| Previous radiation | 112 (54.37) | 69 (52.27) | 43 (58.11) | .467 | 79 (52.32) | 31 (62.00) | .255 |
| Previous blinatumomab | 25 (12.1%) | 17 (12.9%) | 8 (10.8%) | .820 | 17 (11.3%) | 7 (14.0%) | .620 |
| Previous inotuzumab | 17 (8.3%) | 14 (10.6%) | 3 (4.1%) | .120 | 14 (9.3%) | 3 (6.0%) | .570 |
| CAR T-cell product, n (%) | .001 | .660 | |||||
| CTL019 (trial) | 128 (62.1) | 83 (62.9) | 45 (60.8) | 95 (62.9) | 29 (58.0) | ||
| huCART19 (trial) | 33 (16.0) | 13 (9.8) | 20 (27.0) | 22 (14.6) | 10 (20.0) | ||
| Kymriah (commercial) | 45 (21.8) | 36 (27.3) | 9 (12.2) | 34 (22.5) | 11 (22.0) | ||
| Clinical trial or commercial | .008 | .555 | |||||
| Clinical trial | 161 (78.2%) | 96 (72.7) | 65 (87.8) | 117 (77.5%) | 39 (78.0%) | ||
| Commercial product | 45 (21.8%) | 36 (27.3) | 9 (12.2) | 34 (22.5%) | 11 (22.0%) | ||
| Year of infusion, n (%) | .520 | .830 | |||||
| 2012 and 2013 | 22 (10.7) | 18 (13.6) | 4 (5.4) | 19 (12.6) | 3 (6.0) | ||
| 2014 | 23 (11.2) | 13 (9.8) | 10 (13.5) | 17 (11.3) | 5 (10.0) | ||
| 2015 | 25 (12.1) | 17 (12.9) | 8 (10.8) | 18 (11.9) | 7 (14.0) | ||
| 2016 | 31 (15.0) | 19 (14.4) | 12 (16.2) | 23 (15.2) | 6 (12.0) | ||
| 2017 | 51 (24.8) | 29 (22.0) | 22 (29.7) | 36 (23.8) | 13 (26.0) | ||
| 2018 | 38 (18.4) | 25 (18.9) | 13 (17.6) | 26 (17.2) | 12 (24.0) | ||
| 2019 and 2020 | 16 (7.8) | 11 (8.3) | 5 (6.8) | 12 (7.9) | 4 (8.0) | ||
| . | Total (N = 206) . | Insurance status . | Neighborhood opportunity∗ . | ||||
|---|---|---|---|---|---|---|---|
| Private insurance (N = 132) . | Medicaid (N = 74) . | P . | High COI (N = 151) . | Low COI (N = 50) . | P . | ||
| Age categories, n (%) | .170 | .070 | |||||
| <5 y | 23 (11.2) | 15 (11.4) | 8 (10.8) | 19 (12.6) | 3 (6.0) | ||
| 5-9.99 y | 57 (27.7) | 30 (22.7) | 27 (36.5) | 35 (23.2) | 19 (38.0) | ||
| 10 to 17.99 y | 89 (43.2) | 63 (47.7) | 26 (35.1) | 65 (43.0) | 23 (46.0) | ||
| ≥18 | 37 (18.0) | 24 (18.2) | 13 (17.6) | 32 (21.2) | 5 (10.0) | ||
| Sex, n (%) | .770 | .620 | |||||
| Male | 120 (58.3) | 78 (59.1) | 42 (56.8) | 91 (60.3) | 28 (56.0) | ||
| Female | 86 (41.7) | 54 (40.9) | 32 (43.2) | 60 (39.7) | 22 (44.0) | ||
| Race and ethnicity, n (%) | <.001 | <.001 | |||||
| White non-Hispanic | 131 (63.6) | 101 (76.5) | 30 (40.5) | 109 (72.2) | 18 (36.0) | ||
| Black or African American | 15 (7.3) | 6 (4.5) | 9 (12.2) | 10 (6.6) | 5 (10.0) | ||
| Hispanic | 44 (21.4) | 13 (9.8) | 31 (41.9) | 20 (13.2) | 23 (46.0) | ||
| Other | 16 (7.8) | 12 (9.1) | 4 (5.4) | 12 (7.9) | 4 (8.0) | ||
| Cytogenetics category, n (%)† | .560 | .800 | |||||
| Favorable | 42 (20.4) | 29 (22.0) | 13 (17.6) | 34 (22.5) | 8 (16.0) | ||
| Intermediate risk | 24 (11.7) | 14 (10.6) | 10 (13.5) | 15 (9.9) | 7 (14.0) | ||
| High risk | 63 (30.6) | 37 (28.0) | 26 (35.1) | 45 (29.8) | 17 (34.0) | ||
| Uninformative | 71 (34.5) | 49 (37.1) | 22 (29.7) | 52 (34.4) | 17 (34.0) | ||
| Missing | 6 (2.9) | 3 (2.3) | 3 (4.1) | 5 (3.3) | 1 (2.0) | ||
| Disease status at referral, n (%) | .026 | .740 | |||||
| Primary refractory | 33 (16.0) | 28 (21.2) | 5 (6.8) | 25 (16.6) | 8 (16.0) | ||
| First relapse | 62 (30.1) | 35 (26.5) | 27 (36.5) | 47 (31.1) | 13 (26.0) | ||
| Second relapse | 77 (37.4) | 50 (37.9) | 27 (36.5) | 53 (35.1) | 22 (44.0) | ||
| Third or greater relapse | 34 (16.5) | 19 (14.4) | 15 (20.3) | 26 (17.2) | 7 (14.0) | ||
| Marrow status preinfusion, n (%) | .010 | .049 | |||||
| <0.01% | 76 (36.9) | 38 (28.8) | 38 (51.4) | 49 (32.5) | 26 (52.0) | ||
| 0.01% to 4.99% | 42 (20.4) | 33 (25.0) | 9 (12.2) | 35 (23.2) | 6 (12.0) | ||
| 5% to 24.99% | 16 (7.8) | 11 (8.3) | 5 (6.8) | 11 (7.3) | 5 (10.0) | ||
| >25% | 72 (35.0) | 50 (37.9) | 22 (29.7) | 56 (37.1) | 13 (26.0) | ||
| Previous HSCT | 88 (42.7%) | 53 (40.2%) | 35 (47.3%) | .380 | 63 (41.7%) | 23 (46.0%) | .620 |
| Previous radiation | 112 (54.37) | 69 (52.27) | 43 (58.11) | .467 | 79 (52.32) | 31 (62.00) | .255 |
| Previous blinatumomab | 25 (12.1%) | 17 (12.9%) | 8 (10.8%) | .820 | 17 (11.3%) | 7 (14.0%) | .620 |
| Previous inotuzumab | 17 (8.3%) | 14 (10.6%) | 3 (4.1%) | .120 | 14 (9.3%) | 3 (6.0%) | .570 |
| CAR T-cell product, n (%) | .001 | .660 | |||||
| CTL019 (trial) | 128 (62.1) | 83 (62.9) | 45 (60.8) | 95 (62.9) | 29 (58.0) | ||
| huCART19 (trial) | 33 (16.0) | 13 (9.8) | 20 (27.0) | 22 (14.6) | 10 (20.0) | ||
| Kymriah (commercial) | 45 (21.8) | 36 (27.3) | 9 (12.2) | 34 (22.5) | 11 (22.0) | ||
| Clinical trial or commercial | .008 | .555 | |||||
| Clinical trial | 161 (78.2%) | 96 (72.7) | 65 (87.8) | 117 (77.5%) | 39 (78.0%) | ||
| Commercial product | 45 (21.8%) | 36 (27.3) | 9 (12.2) | 34 (22.5%) | 11 (22.0%) | ||
| Year of infusion, n (%) | .520 | .830 | |||||
| 2012 and 2013 | 22 (10.7) | 18 (13.6) | 4 (5.4) | 19 (12.6) | 3 (6.0) | ||
| 2014 | 23 (11.2) | 13 (9.8) | 10 (13.5) | 17 (11.3) | 5 (10.0) | ||
| 2015 | 25 (12.1) | 17 (12.9) | 8 (10.8) | 18 (11.9) | 7 (14.0) | ||
| 2016 | 31 (15.0) | 19 (14.4) | 12 (16.2) | 23 (15.2) | 6 (12.0) | ||
| 2017 | 51 (24.8) | 29 (22.0) | 22 (29.7) | 36 (23.8) | 13 (26.0) | ||
| 2018 | 38 (18.4) | 25 (18.9) | 13 (17.6) | 26 (17.2) | 12 (24.0) | ||
| 2019 and 2020 | 16 (7.8) | 11 (8.3) | 5 (6.8) | 12 (7.9) | 4 (8.0) | ||
Five patients had addresses that could not be geocoded, thus removed in all COI analyses.
High-risk lesions defined as KMT2A (MLL) rearrangements, Philadelphia-chromosome, Ph-like, hypodiploid (<44 chromosomes), and TCF3/HLF fusion. Intermediate-risk lesions included iAMP21, IKZF1 deletion, or TCF3/PBX1. Favorable cytogenetics were defined as the presence of hyperdiploidy (>51 chromosomes), or ETV6/RUNX1 fusion. Any other lesions not specified were considered uninformative based on prior analysis of cytogenetics and CAR T-cell therapy outcomes.
The distribution of covariates was similar when examining COI in quintiles (supplemental Table 1). Locally referred patients (18%) did not differ from externally referred patients (82%) by household poverty, neighborhood opportunity, race and ethnicity, or disease burden at the time of infusion (supplemental Table 2).
Overall survival
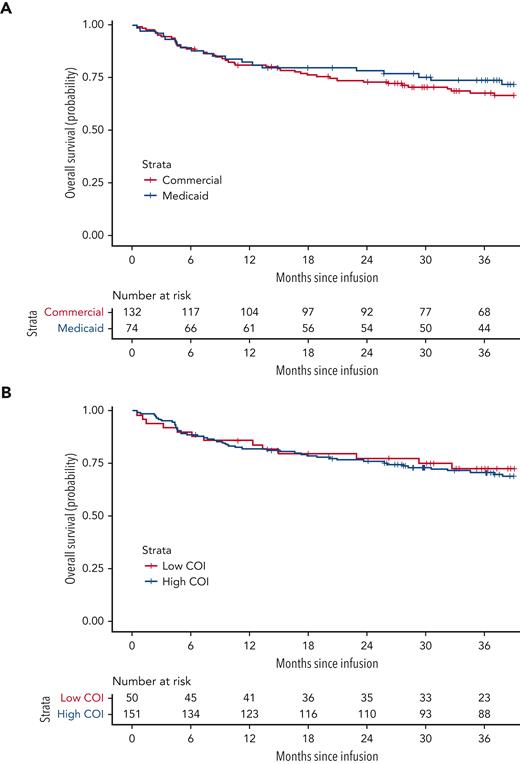
OS did not differ significantly by household poverty exposure (P = .483, 36-month OS: 73.8% [95% confidence interval (CI), 64.3-84.7] household poverty–exposed, 67.7% [95% CI, 60.0-76.4] household poverty–unexposed; Figure 2A). Multivariate analysis adjusting for age, race and ethnicity, disease status, CAR T-cell product (CTL019 vs huCART19), prior inotuzumab, and disease burden at the time of infusion further demonstrated a comparable hazard of death by household poverty exposure (adjusted HR, 1.0; 95% CI, 0.5-2.0) (Table 2).
OS by household-poverty exposure and neighborhood opportunity. (A) OS by household poverty exposure (proxied by insurance status), defined as time from infusion to date of death from any cause, log-rank P = .483. (B) OS by neighborhood opportunity, log-rank P = .724.
OS by household-poverty exposure and neighborhood opportunity. (A) OS by household poverty exposure (proxied by insurance status), defined as time from infusion to date of death from any cause, log-rank P = .483. (B) OS by neighborhood opportunity, log-rank P = .724.
Univariate and multivariate Cox regression for OS and RFS by insurance status and COI
| . | OS (n = 206)∗ . | RFS (n = 192)† . | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) . | P . | Adjusted HR (95% CI) . | P . | Unadjusted HR (95% CI) . | P . | Adjusted HR (95% CI) . | P . | |
| Insurance‡ | ||||||||
| Commercial | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Medicaid | 0.8 (0.5-1.4) | 0.484 | 1.0 (0.5-2.0) | 0.971 | 0.8(0.5-1.3) | 0.452 | 0.7 (0.4-1.3) | 0.273 |
| COI§ | ||||||||
| High | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Low | 0.9 (0.5-1.7) | 0.724 | 1.2 (0.6-2.4) | 0.545 | 1.5 (0.9-2.4) | 0.104 | 2.3 (1.3-4.1) | 0.006 |
| COI health/environment|| | ||||||||
| High | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Low | 1.1 (0.6-1.9) | 0.780 | 1.1 (0.6-2.0) | 0.851 | 1.1 (0.7-1.9) | 0.658 | 1.4 (0.8-2.5) | 0.296 |
| COI education¶ | ||||||||
| High | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Low | 1.0 (0.6-1.8) | 0.997 | 1.2 (0.6-2.3) | 0.636 | 1.2 (0.7-2.0) | 0.445 | 1.6 (0.9-3.0) | 0.107 |
| COI social/economic§ | ||||||||
| High | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Low | 0.7 (0.4-1.3) | 0.277 | 0.8 (0.4-1.7) | 0.626 | 1.3 (0.8-2.2) | 0.276 | 1.6 (0.9-2.8) | 0.105 |
| . | OS (n = 206)∗ . | RFS (n = 192)† . | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) . | P . | Adjusted HR (95% CI) . | P . | Unadjusted HR (95% CI) . | P . | Adjusted HR (95% CI) . | P . | |
| Insurance‡ | ||||||||
| Commercial | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Medicaid | 0.8 (0.5-1.4) | 0.484 | 1.0 (0.5-2.0) | 0.971 | 0.8(0.5-1.3) | 0.452 | 0.7 (0.4-1.3) | 0.273 |
| COI§ | ||||||||
| High | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Low | 0.9 (0.5-1.7) | 0.724 | 1.2 (0.6-2.4) | 0.545 | 1.5 (0.9-2.4) | 0.104 | 2.3 (1.3-4.1) | 0.006 |
| COI health/environment|| | ||||||||
| High | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Low | 1.1 (0.6-1.9) | 0.780 | 1.1 (0.6-2.0) | 0.851 | 1.1 (0.7-1.9) | 0.658 | 1.4 (0.8-2.5) | 0.296 |
| COI education¶ | ||||||||
| High | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Low | 1.0 (0.6-1.8) | 0.997 | 1.2 (0.6-2.3) | 0.636 | 1.2 (0.7-2.0) | 0.445 | 1.6 (0.9-3.0) | 0.107 |
| COI social/economic§ | ||||||||
| High | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Low | 0.7 (0.4-1.3) | 0.277 | 0.8 (0.4-1.7) | 0.626 | 1.3 (0.8-2.2) | 0.276 | 1.6 (0.9-2.8) | 0.105 |
Ref, reference.
COI analyses include 201 patients, removing patients missing COI.
COI analyses include 188 patients, removing patients missing COI and not in CR.
Multivariate model adjusted for: age, race and ethnicity, disease status, prior inotuzumab, product, disease burden.
Multivariate model adjusted for: age, race and ethnicity, disease burden.
Multivariate model adjusted for: age, race and ethnicity, disease status, cytogenetics, disease burden.
Multivariate model adjusted for: age, sex, race and ethnicity, relapse status, cytogenetics, disease burden.
OS also did not differ significantly by neighborhood opportunity (P = .724, 36-month OS: 72.6% [95% CI, 60.8-86.6] low-neighborhood opportunity vs 70.7% [95% CI, 63.7-78.5] high-neighborhood opportunity; Figure 2B). Following adjustment for age, race and ethnicity, and disease burden at the time of infusion, the hazard of death was similar for patients exposed to low- vs high-opportunity neighborhoods (adjusted HR, 1.2; 95% CI, 0.6-2.4). In an exploratory, multivariable adjusted comparison of low- vs high-opportunity in the educational, social/economic, and the health/environment COI domains, there were also no significant differences in hazard of death (Table 2).
Relapse free survival
Two patients died before the first evaluation at day 28 after CAR T-cell infusion, and 12 patients did not achieve CR and were excluded from the RFS analysis. Overall, the CR rate for the cohort was 93.2%. There was no significant difference in CR by household poverty (poverty-exposed 91.9% vs poverty-unexposed 93.9% vs P = .334) or neighborhood opportunity (low 90.0% vs high 94.7% vs; P = .504).
RFS was not significantly different by household poverty exposure (36-month RFS 61.0% [95% CI, 49.6-75.2] household poverty–exposed vs 52.5% [95% CI, 49.0-69.1] unexposed P = .361; Figure 3A). In multivariable adjusted analysis, there was no statistically significant difference in RFS by household poverty exposure (adjusted HR, 0.7; 95% CI, 0.4-1.3; P = .273) (Table 2).
RFS by household poverty exposure and neighborhood opportunity. (A) Displays RFS by household poverty (proxied by insurance status), defined as time from onset of remission to relapse in the patients who achieved CR after infusion, log-rank P = .3610. (B) Displays RFS by neighborhood opportunity, log-rank P = .2550. Data were censored for allogeneic HSCT (n = 22), alternative therapy during remission (n = 21), or subsequent malignancy (n = 2).
RFS by household poverty exposure and neighborhood opportunity. (A) Displays RFS by household poverty (proxied by insurance status), defined as time from onset of remission to relapse in the patients who achieved CR after infusion, log-rank P = .3610. (B) Displays RFS by neighborhood opportunity, log-rank P = .2550. Data were censored for allogeneic HSCT (n = 22), alternative therapy during remission (n = 21), or subsequent malignancy (n = 2).
In contrast, RFS was worse for low-opportunity vs high-opportunity neighborhood exposure. However, this did not achieve statistical significance in unadjusted analyses (36-month RFS 46.5% [95% CI, 32.6-66.3] low-opportunity neighborhood vs 60.1% [95% CI, 51.5-70.1] high-opportunity neighborhood, P = .255; Figure 3B). Following multivariable adjustment, patients from low-opportunity neighborhoods had a significantly higher hazard of relapse compared with those from high-opportunity neighborhoods (adjusted HR, 2.3; 95% CI, 1.3-4.1; P = .006).
In exploratory analyses examining low-neighborhood opportunity in each of the COI domains, low-neighborhood opportunity was consistently associated with a higher hazard of relapse in the educational domain (HR, 1.6; 95% CI, 0.9-3.0; P = .107) and the social/economic domain (HR, 1.6; 95% CI, 0.9-2.8; P = .105), though none reached the threshold for statistical significance (Table 2).
To investigate variability in types of events (CD19+ or CD19- relapse, or death without relapse) and types of censoring (HSCT, other alternative cancer-directed therapy, subsequent malignancy, or last contact), frequency of the first event was summarized by household poverty and neighborhood opportunity exposures (Table 3). Type of event varied by exposure group, with CD19+ relapse more frequent in the low-neighborhood opportunity cohort as compared with the high-neighborhood opportunity cohort (24.4% vs 12.6%). Type of censoring events also varied by exposure, with HSCT less frequent among household poverty–exposed (8.8% vs 12.9%) and low-neighborhood opportunity–exposed patients (8.9% vs 12.6%). Use of alternative cancer-directed therapy (including conventional chemotherapy, other CAR T-cell therapy, and tyrosine kinase inhibitor) as a censoring event was also less frequent among household poverty–exposed (7.4% vs 12.9%) and low-COI–exposed patients (6.7% vs 11.9%).
Details on types of events and censoring in RFS analyses
| . | Insurance status . | COI . | ||
|---|---|---|---|---|
| Commercial (n = 124), n (%) . | Medicaid (n = 68), n (%) . | High COI (n = 143), n (%) . | Low COI (n = 45), n (%) . | |
| Events | ||||
| Relapse CD 19+ | 18 (14.52) | 11 (16.18) | 18 (12.59) | 11 (24.44) |
| Relapse CD 19- or lineage switch | 28 (22.58) | 14 (20.59) | 29 (20.28) | 8 (17.77) |
| Death without relapse | 1 (0.81) | 1 (1.47) | 0 (0) | 2 (1.40) |
| Censoring | ||||
| HSCT | 16 (12.90) | 6 (8.82) | 18 (12.59) | 4 (8.89) |
| Alternative cancer-therapy∗ | 16 (12.90) | 5 (7.35) | 17 (11.89) | 3 (6.67) |
| Subsequent malignancy | 2 (1.61) | 0 (0) | 0 (0) | 2 (4.44) |
| Last contact | 43 (34.68) | 31 (45.59) | 59 (41.26) | 15 (33.33) |
| . | Insurance status . | COI . | ||
|---|---|---|---|---|
| Commercial (n = 124), n (%) . | Medicaid (n = 68), n (%) . | High COI (n = 143), n (%) . | Low COI (n = 45), n (%) . | |
| Events | ||||
| Relapse CD 19+ | 18 (14.52) | 11 (16.18) | 18 (12.59) | 11 (24.44) |
| Relapse CD 19- or lineage switch | 28 (22.58) | 14 (20.59) | 29 (20.28) | 8 (17.77) |
| Death without relapse | 1 (0.81) | 1 (1.47) | 0 (0) | 2 (1.40) |
| Censoring | ||||
| HSCT | 16 (12.90) | 6 (8.82) | 18 (12.59) | 4 (8.89) |
| Alternative cancer-therapy∗ | 16 (12.90) | 5 (7.35) | 17 (11.89) | 3 (6.67) |
| Subsequent malignancy | 2 (1.61) | 0 (0) | 0 (0) | 2 (4.44) |
| Last contact | 43 (34.68) | 31 (45.59) | 59 (41.26) | 15 (33.33) |
HSCT, chemotherapy, other immunotherapy or CAR T-cell therapy, tyrosine kinase inhibitors.
Sensitivity analyses
Sensitivity analyses exploring outcomes by COI in quintiles (supplemental Table 3), excluding race and ethnicity from the multivariate model, and adjusting for additional covariates associated with censoring (disease status, prior radiation, year of infusion, and CAR T-cell product [CTL019, huCART19, tisagenlecleucel]) all mirrored results from the primary analysis (supplemental Table 4).
In a sensitivity analysis exploring time to relapse or death with HSCT/alternative cancer-directed therapy and subsequent malignancy as competing risks, the cumulative incidence of relapse or death was not significantly different by household poverty exposure (Gray P = .685; supplemental Figure 2A) or neighborhood opportunity (Gray P = .175; supplemental Figure 2B). Although not statistically significant, patients unexposed to household poverty (Gray P = .114 ) and those from high-opportunity neighborhoods (Gray P = .218) had a higher cumulative incidence of HSCT/alternative cancer-directed therapy: 36-month cumulative incidence is 16.2% (95% CI, 8.6-25.9) household poverty–exposed vs 25% (95% CI, 18.6-34.0) household poverty–unexposed; 15.6% (95% CI, 6.8-27.8) low-neighborhood opportunity vs 24.6% (95% CI, 17.8-31.9) high-neighborhood opportunity. The cause-specific hazard models (univariate and multivariate) for relapse or death with HSCT/alternative cancer-directed therapy and subsequent malignancy treated as competing risks yielded results comparable to the primary Cox regression analyses presented in Table 2.
Discussion
Neither household poverty nor low-neighborhood opportunity are associated with CR or OS in a cohort of children with ALL or LLy who received CAR T-cell therapy at a tertiary care center. This finding is contrary to evidence of persistent disparities in the context of other highly resourced, centralized therapies, including HSCT for hematologic malignancy14 and immunotherapy for neuroblastoma.15
Unlike frontline therapy for ALL, which requires 2 years of therapy, including adherence to home oral chemotherapy and frequent physician dose adjustment for blood counts, CAR T-cell therapy is given as a single infusion, potentially ameliorating disparities related to physician dosing practices33 or oral chemotherapy adherence.34,35 After CAR T-cell therapy, patients experience B-cell aplasia and require monthly intravenous immunoglobulin, for which access may be challenging. This represents another important area for future investigation and intervention.
We observed an increased hazard of relapse among patients from low-opportunity neighborhoods, although OS was similar, potentially suggesting superior salvageability in this cohort. This may be related to CD19 status: low-neighborhood opportunity patients were more likely to experience CD19+ relapse when compared with high-neighborhood opportunity patients who relapsed, possibly attributable to a lower disease burden at infusion36 among low-neighborhood opportunity patients, as CD19 positivity allows for salvage with additional CD19-targeted agents. This increased hazard of relapse was not observed among household poverty–exposed patients. Notably, our proxied exposures identified distinct cohorts: only 60% of low-neighborhood opportunity patients were household poverty–exposed, and 27% of high-neighborhood opportunity patients were household poverty–exposed. Consequently, unmeasured social determinants of health exposures at the neighborhood-level, not explained by neighborhood opportunity alone, may influence the risk of relapse but not affect salvageability. Future studies must examine more granular area–based and household-level exposures with a larger, multi-institutional cohort and specifically explore family-reported household-level poverty exposures (or adverse social determinants of health [SDOH]) to overcome the limitations of proxied SDOH that result in misclassification bias.
Our data demonstrate a difference in disease burden at the time of referral for CAR T-cell infusion, such that children from more advantaged households (unexposed to household poverty or living in high-opportunity neighborhoods) were significantly more likely to present with a high disease burden. This is notable given that a higher disease burden at the time of CAR T-cell therapy is associated with inferior outcome and a greater risk of toxicity,24,37,38 and thus not the ideal status for CAR T-cell therapy referral. It is possible that this difference could indicate inequity in access or bias in referral patterns, with providers less willing to recommend sicker patients of lower socioeconomic status travel to a tertiary care center for therapy. Alternatively, socioeconomically advantaged families may have greater ability to both advocate for CAR T-cell therapy and travel to an accredited cell therapy center, even in the context of more severe disease. In addition, our results suggest that children from more advantaged households may be more likely to receive alternative therapy (including HSCT) following CAR T-cell infusion before relapse. This may be related to household poverty–unexposed patients, and high-neighborhood opportunity patients entering cell therapy with a greater disease burden, heightening provider concern for a potential relapse. Although these interventions did not lead to superior outcomes, they potentially reflect bias in practice variability or family advocacy based on socioeconomic advantage.
Our study is limited by its single-center nature, and findings cannot be generalized to all CAR T-cell therapy centers. The CHOP Cell Therapy Program is well resourced and offers support for travel and housing during the first month after infusion, a supportive care approach that may have been biased toward the null by mitigating barriers to access. Furthermore, our study cohort included only 15 Black patients (7.28%), which may suggest underrepresentation of this historically marginalized population based on previous clinical trials for r/r leukemia,39 potentially highlighting an access disparity, which has also been observed in the adult setting,40 and limiting generalizability. In addition, we used insurance at the time of CAR T-cell therapy to proxy household poverty exposure owing to the nonavailability of family-reported household poverty exposures (eg, income, food insecurity), and this approach may have led to misclassification bias. Specifically, this patient population is a heavily pretreated cohort, and childhood cancer affects parental finances and employment;41 consequently, children from higher-income homes may have lost private insurance by the time of CAR T-cell therapy and thus been misclassified as household poverty–exposed. Notably, most patients on this study were also treated with investigational agents, and thus findings cannot be generalized to all commercial product administration. Future studies should incorporate self-reported income and other self-reported social determinants of health over time.
Treatment with CTL019, huCART19, or tisagenlecleucel produces durable remission and equivalent survival for children with r/r B-ALL and LLy, with a 3-year OS rate of ∼70%, regardless of household poverty or neighborhood opportunity. This finding suggests that CAR T-cell therapy could potentially ameliorate some of the factors influencing the disparate outcomes observed in other treatment settings for children with ALL. Despite this, evidence of potential bias in access or referral patterns based on disease burden suggests opportunities for interventions to improve access for less socioeconomically advantaged children with high disease burden and minority patients who may still benefit from CAR T-cell therapy. Multi-institutional studies outside of clinical trial settings using patient-reported and neighborhood-level social determinants of health for further investigation of outcomes and toxicities are critical avenues for future research.
Acknowledgment
The authors thank the Cancer Immunotherapy Program clinical research team at the Children’s Hospital of Philadelphia. This study was supported by the Children’s Hospital of Philadelphia Frontier Program. H.N, R.M.M., S.L.M., A.B.L., and S.A.G. are supported by grants from the National Institutes of Health (5T32CA009615 to H.N. 5P01CA214278–04 to S.A.G. and S.L.M., 5K12CA076931–22 to R.M.M and A.B.L.).
Authorship
Contribution: H.N., A.B.L., K.B., Y.L., and H.L. were involved in the conception, design, and planning of the study; H.N., A.B.L., R.M.M., A.D., L.W., S.R.R., C.C., C.W., D.B., S.A.G., and S.L.M. collected the data; V.T. performed geocoding; Y.L., H.L., and H.N. performed the statistical analysis; and all authors reviewed the data analyses, contributed to data interpretation and writing of the report, and approved the final version of the submitted report.
Conflict-of-interest disclosure: C.C. has served as a consultant for Novartis Pharmaceuticals. S.A.G. has received research and/or clinical trial support from Novartis, Servier, and Kite and has participated in consulting, study steering committees, or scientific/clinical advisory boards for Novartis, Cellectis, Adaptimmune, Eureka, TCR2, Juno, GlaxoSmithKline, Vertex, Cure Genetics, Humanigen, and Roche. S.R.R. received research funding and has served as a consultant for Pfizer, Inc. S.L.M. reports clinical trial support and fees for consultancy and advisory boards from Novartis Pharmaceuticals and Wugen, and a patent for PCT/US2017/044425: Combination Therapies of Car and PD-1 Inhibitors pending and licensed to Novartis Pharmaceuticals. C.H.J. is an inventor of tisagenlecleucel and receives royalties from Novartis. D.T.T. serves on advisory boards for Sobi, Janssen, and BEAM Therapeutics. D.T.T. receives research funding from BEAM Therapeutics and NeoImmune Tech. D.T.T. has patents pending on CD38 CAR T cells for hematologic malignancies and biomarkers for cytokine release syndrome.
Correspondence: Allison Barz Leahy, Children’s Hospital of Philadelphia, The Hub for Clinical Collaboration, Division of Oncology, 3rd Floor, Rm 3548, 3500 Civic Center Blvd, Philadelphia, PA 19104; e-mail: barza@chop.edu.
References
Author notes
Data are available on request from the corresponding author, Allison Barz Leahy (barza@chop.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
K.B. and A.B.L. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal