Key Points
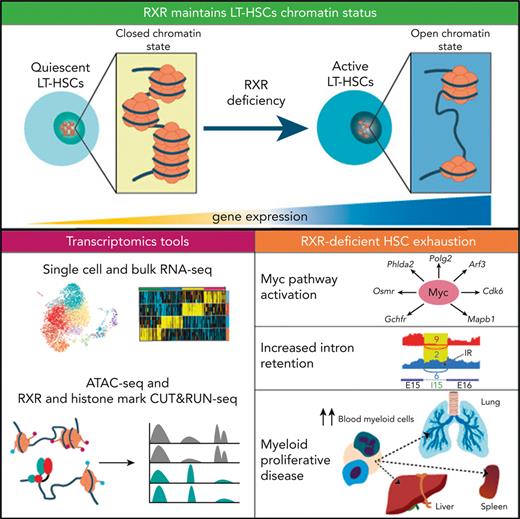
RXRs are ligand-activated transcriptional units needed for the maintenance of HSC fitness, and preservation of a balanced hematopoiesis.
RXRα;RXRβ-deficient HSCs transition from a dormant to a transcriptionally and proliferative-active state through MYC pathway activation.
Abstract
Hematopoietic stem cells (HSCs) balance self-renewal and differentiation to maintain hematopoietic fitness throughout life. In steady-state conditions, HSC exhaustion is prevented by the maintenance of most HSCs in a quiescent state, with cells entering the cell cycle only occasionally. HSC quiescence is regulated by retinoid and fatty-acid ligands of transcriptional factors of the nuclear retinoid X receptor (RXR) family. Herein, we show that dual deficiency for hematopoietic RXRα and RXRβ induces HSC exhaustion, myeloid cell/megakaryocyte differentiation, and myeloproliferative-like disease. RXRα and RXRβ maintain HSC quiescence, survival, and chromatin compaction; moreover, transcriptome changes in RXRα;RXRβ-deficient HSCs include premature acquisition of an aging-like HSC signature, MYC pathway upregulation, and RNA intron retention. Fitness loss and associated RNA transcriptome and splicing alterations in RXRα;RXRβ-deficient HSCs are prevented by Myc haploinsufficiency. Our study reveals the critical importance of RXRs for the maintenance of HSC fitness and their protection from premature aging.
Introduction
Hematopoietic stem cell (HSC) self-renewal and differentiation are subject to tight transcriptional regulation.1 Features, such as altered transcriptional programs, cellular stress, or age, can impair HSC fitness and performance.2,3 HSC fitness loss and exhaustion are associated with a gradual decline in HSC regenerative capacity, leading to an expansion of functionally weakened HSCs with an altered multilineage differentiation potential.4,5
Retinoid X receptors (RXRs) are members of the nuclear receptor (NR) superfamily of ligand-dependent transcription factors (TFs)6 that respond to vitamin A derivatives (retinoids) and some endogenous fatty acids (FAs).7 RXRs are encoded by 3 genes, Rxra (NR2B1), Rxrb (NR2B2), and Rxrg (NR2B3), which show time-specific and tissue-dependent differential expression. RXRs control pleiotropic genetic programs, such as cell proliferation and differentiation, immune functions, and lipid and glucose metabolism, by forming homodimers and heterodimers with others NRs, including other retinoid-activated receptors, the retinoic acid receptors (RARs).6 Retinoid-activated receptors are an attractive therapeutic target because of their ability to induce neutrophil differentiation.8-10 However, their specific role in HSC maintenance and survival remains controversial, with conflicting results obtained with mice vs human HSCs.10-13 Recent studies with retinoid-supplementation and retinoid-deprivation models demonstrated that retinoid signaling is essential for the regulation of HSC dormancy.5,14 Because retinoids activate both the RARs and RXRs, some of the effects observed in these studies are likely driven by RXRs. Pharmacologic approaches using RXR ligands suggest the importance of RXRs in HSC self-renewal and myeloid differentiation in vitro,15,16 although off-target effects and the inability to generate complete loss-of-function models have hampered the interpretation of these experiments. To date, no definitive genetic evidence has confirmed the role of the RXRs in HSC activity, because deficiency of individual RXR isoforms results in compensatory activity.17 Herein, we demonstrate that double deletion of Rxra and Rxrb leads to a decline in HSC fitness, characterized by HSC proliferative activation and exhaustion, and concomitant development of myeloproliferative-like disease (MPD). This phenotype derives from loss of HSC quiescence and self-renewal, openness of HSC chromatin, aberrant splicing with increased intron retention, and activation of the signaling pathway controlled by MYC.18 Using a model of Myc haploinsufficiency, we demonstrate that the loss of quiescence and altered transcription and splicing in RXR-deficient HSCs are mediated by MYC pathway activation. Our results provide definitive evidence that RXRs regulate HSC fitness and lineage fate by preserving transcriptional repression of the self-renewal master regulator MYC, and open new perspectives on the development of therapeutic approaches to prevent or reverse loss of HSC fitness.
Materials and methods
Mice
Details of the generation and crossings of the animals used are provided in supplemental Materials and Methods, available on the Blood website.
Cell isolation
Cell isolation procedures are detailed in supplemental Materials and Methods.
Flow cytometry and cell sorting
Procedures for flow cytometry and cell sorting are described in supplemental Materials and Methods.
Colony-forming unit cell assays
Colony-forming unit (CFU) cell assays were performed as detailed in supplemental Materials and Methods.
OP9NL1 cocultures
OP9NL1 cocultures were performed as detailed in supplemental Materials and Methods.
Generation of hematopoietic chimeras
Hematopoietic chimeras were generated as detailed in supplemental Materials and Methods.
Homing assays
The procedures for homing assays are detailed in supplemental Materials and Methods.
5-Fluorouracil assay
The 5-fluorouracil (5-FU) assays were performed as detailed in supplemental Materials and Methods.
Immunohistochemistry
Immunohistochemistry approaches are detailed in supplemental Materials and Methods.
HSC first division fate analysis
The procedures for first division fate analysis are described in supplemental Materials and Methods.
Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction experiments were performed as detailed in supplemental Materials and Methods.
Single-cell RNA-sequencing processing and analysis
The 10× Genomics Chromium 3′ v2 assay standard protocols were as previously described,19 with minor modifications detailed in supplemental Materials and Methods.
Bulk, deep RNA-sequencing processing and analysis
Bulk, deep RNA-sequencing assays are detailed in supplemental Materials and Methods.
Chromatin profiling by assay for transposase-accessible chromatin with sequencing
Assay for transposase-accessible chromatin with sequencing was done as previously described,20 with minor modifications detailed in supplemental Materials and Methods.
Cleavage under target and release using nuclease sequencing assay
Cleavage under target and release using nuclease sequencing (CUT&RUNseq) assays were performed following published methods21 with minor modifications, as detailed in supplemental Materials and Methods.
Serial competitive repopulation of Myc haploinsufficient HSCs
Serial competitive repopulation assays are described in supplemental Materials and Methods.
Statistical analysis
Statistical procedures are described in supplemental Materials and Methods.
Results
Dual deletion of RXRα and RXRβ in the hematopoietic compartment causes expansion of the multipotential and myeloid progenitor cell pools
Transcript expression analysis of bone marrow (BM) hematopoietic cell subsets revealed ubiquitous Rxra and Rxrb expression in hematopoietic stem and multipotential progenitor cells (HSC/Ps; lineage−Sca-1+c-kit+ cells; LSKs), including long-term (LT) HSCs, short-term HSCs, and multipotent progenitors (MPP2 and MPP3/MPP4),22 as well as in committed erythromyeloid progenitors (lineage−Sca-1−c-kit+ cells; LKs), including common myeloid, granulocyte-monocyte, and erythroid-megakaryocyte progenitors (supplemental Figure 1A-B). In all these hematopoietic populations, Rxrg expression was undetectable (qPCR cycle threshold [CT] values ≥35). We inactivated RXRα or RXRβ in the HSC/Ps of neonatal polyinosinic:polycytidylic acid (pI:pC)-inducible Mx-1Cre transgenic mice23 (supplemental Figure 2A). Postnatal deletion of RXRα (Mx-1Cre+Rxrafl/fl mice) or RXRβ (Mx-1Cre+Rxrbfl/fl mice) in HSC/Ps was insufficient to substantially alter hematopoiesis (supplemental Figure 2B-G; supplemental Table 1). However, the deletion of both RXR isoforms (Mx-1Cre+Rxrabfl/fl mice) led to paler bones, reduced BM cellularity, splenomegaly, T-cell lymphopenia, and neutrophilia (supplemental Figure 2A-B; supplemental Table S1). Mx-1Cre+Rxrabfl/fl mice also had significantly elevated numbers of eosinophils and LSKs in BM, reduced T-cell numbers in peripheral blood (PB) and spleen, and significantly elevated numbers of spleen LSKs, LKs, and mature myeloid cells (supplemental Figure 2C-G). Thus, lack of RXRα;RXRβ in HSC/Ps leads to an expansion of multipotential and myeloid progenitor populations and to lymphopenia.
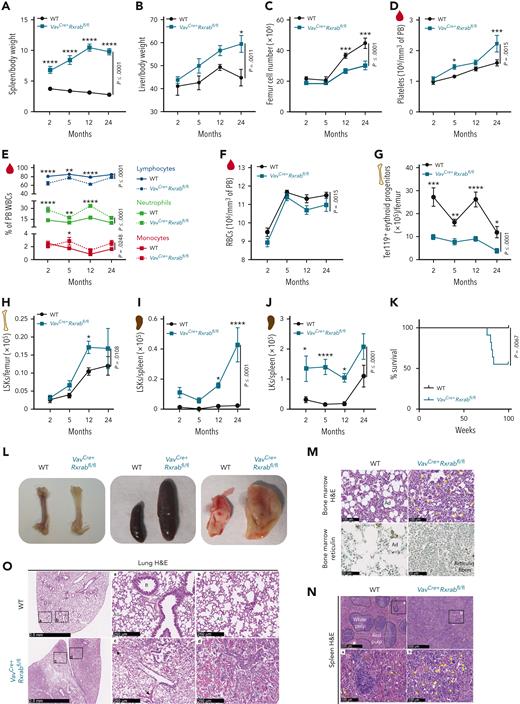
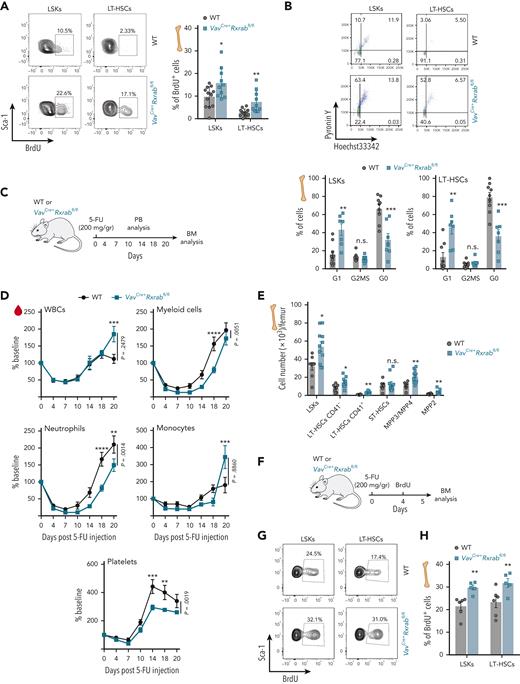
RXRα;RXRβ-deficient mice develop fatal myeloproliferative disease, featuring prominent extramedullary hematopoiesis
To specifically delete Rxra and Rxrb in the hematopoietic compartment from embryonic stages onwards, we used the pan-hematopoietic VavCre mouse model.24 This resulted in loss of Rxra and Rxrb expression in HSC/Ps (supplemental Figure 3A) with no compensatory upregulation of Rxrg (CT values ≥ 35). Analysis of hematopoietic parameters over 2 years revealed extramedullary hematopoiesis in VavCre+Rxrabfl/fl mice, shown by an early increase in spleen/body weight ratio, a progressive increase in liver/body weight ratio, and a later reduction in BM cellularity (Figure 1A-C; supplemental Figure 3B). PB analysis of young VavCre+Rxrabfl/fl mice revealed thrombocytosis (Figure 1D), lymphocytopenia, neutrophilia, and monocytosis (Figure 1E), as well as mild microcytic anemia (Figure 1F; supplemental Figure 3C) and reduced BM erythropoiesis (Figure 1G; supplemental Figure 3D-E). VavCre+Rxrabfl/fl mice also had significantly increased numbers of BM and spleen LSKs (Figure 1H-I) and of spleen LKs, neutrophils, eosinophils, and monocytes (Figure 1J; supplemental Figure 3F-H). These animals also showed a significant reduction in the numbers of PB B and T lymphocytes and in the CD4:CD8 ratio (supplemental Figure 3I-J). All these hematopoietic alterations persisted, and in most cases were magnified in aged VavCre+Rxrabfl/fl mice (Figure 1A-J; supplemental Figure 3C-J). Approximately half of the VavCre+Rxrabfl/fl mice died at around 80 weeks of age, whereas none of the wild-type (WT) mice had died at the study end point (Figure 1K). Necropsy of surviving 24-month-old VavCre+Rxrabfl/fl mice revealed pale bones, pronounced splenomegaly, and enlarged yellow-milky lungs (Figure 1L). Histologic studies of distal femurs revealed increased numbers of megakaryocytes with hyperlobulated nuclei and an absence of adipocytes, with 50% of VavCre+Rxrabfl/fl mice also presenting increased reticulin fibrosis with no collagen deposition (Figure 1M; supplemental Figure 4A). VavCre+Rxrabfl/fl mice had enlarged spleens with altered architecture and increased numbers of megakaryocytes (Figure 1L,N), as well as an overabundance of liver and lung leukocytes (Figure 1O; supplemental Figure 4B-D). Histopathologic changes in VavCre+Rxrabfl/fl lung tissue included massive alveolar inflammatory infiltration, accumulation of lipid-laden cells, alveolar hemorrhage, and abundant crystal deposition (Figure 1O). Morphologic analysis of PB smears showed no detectable blasts, immature myeloblasts, or erythroid precursors in VavCre+Rxrabfl/fl mice. The histopathologic data of VavCre+Rxrabfl/fl marrow and extramedullary hematopoiesis fulfilled the criteria for murine MPD.25,26
VavCre+Rxrabfl/flmice develop lethal myeloproliferative disorders. (A-C) Organ weight and counts of 2-, 5-, 12-, and 24-month-old WT and VavCre+Rxrabfl/fl mice. (A-B) Spleen and liver weigh/body weight ratios. (C) BM cellularity per femur. (D-F) Hemogram data of 2-, 5-, 12-, and 24-month-old WT and VavCre+Rxrabfl/fl mice. (D) Mean platelet counts. (E) Percentage of lymphocytes, neutrophils, and monocytes among white blood cells (WBCs). (F) Total red blood cells (RBCs). (G-J) Flow cytometry of femur and spleen homogenates from 2-, 5-, 12-, and 24-month-old WT and VavCre+Rxrabfl/fl mice. (G) Absolute numbers of erythroid progenitors (CD45−Ter119+ cells) per femur. (H) Absolute numbers of LSKs per femur. (I-J) Absolute numbers of spleen LSKs and LKs. (K) Kaplan-Meier survival plots for WT and VavCre+Rxrabfl/fl mice (n = 11-13 per genotype). (L) Representative images of femurs, spleens, and lungs of 24-month-old WT and VavCre+Rxrabfl/fl mice. (M-N) Representative images of hematoxylin and eosin (H&E)– and reticulin-stained femur sections, and of H&E-stained spleen sections from 24-month-old WT and VavCre+Rxrabfl/fl mice; yellow arrows indicate megakaryocytes. Scale bars: 500 μm (N, upper panels), 100 μm (M, upper panels; N, bottom panels), or 50 μm (M, bottom panels). (O) Representative images of H&E-stained lungs from 24-month-old WT and VavCre+Rxrabfl/fl mice; black arrows indicate alveolar hemorrhage. Scale bars: 2.5 mm (left panels) or 250 μm (middle and right panels). Data are presented as mean ± SEM: (A-F) n = 7 to 17 mice, pooled from up to 3 independent experiments per age group and genotype; (G) n = 3 to 13 mice, pooled from up to 2 independent experiments per age group and genotype; (H) n = 4 to 24 mice, pooled from up to 3 independent experiments per age group and genotype; (I) n = 5 to 12, pooled from up to 2 independent experiments per age and genotype (spleen); (J) n = 5 to 11 mice, pooled from up to 2 independent experiments per age and genotype. Significance was determined by 2-way analysis of variance followed by the Sidak multiple comparisons test (age-paired mice) (A-J) or log-rank (Mantel-Cox) test (K), and is represented as follows: ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, and ∗∗∗∗P ≤ .0001. A, alveoli; Ad, adipocytes; AS, alveolar sac; B, bronchiole.
VavCre+Rxrabfl/flmice develop lethal myeloproliferative disorders. (A-C) Organ weight and counts of 2-, 5-, 12-, and 24-month-old WT and VavCre+Rxrabfl/fl mice. (A-B) Spleen and liver weigh/body weight ratios. (C) BM cellularity per femur. (D-F) Hemogram data of 2-, 5-, 12-, and 24-month-old WT and VavCre+Rxrabfl/fl mice. (D) Mean platelet counts. (E) Percentage of lymphocytes, neutrophils, and monocytes among white blood cells (WBCs). (F) Total red blood cells (RBCs). (G-J) Flow cytometry of femur and spleen homogenates from 2-, 5-, 12-, and 24-month-old WT and VavCre+Rxrabfl/fl mice. (G) Absolute numbers of erythroid progenitors (CD45−Ter119+ cells) per femur. (H) Absolute numbers of LSKs per femur. (I-J) Absolute numbers of spleen LSKs and LKs. (K) Kaplan-Meier survival plots for WT and VavCre+Rxrabfl/fl mice (n = 11-13 per genotype). (L) Representative images of femurs, spleens, and lungs of 24-month-old WT and VavCre+Rxrabfl/fl mice. (M-N) Representative images of hematoxylin and eosin (H&E)– and reticulin-stained femur sections, and of H&E-stained spleen sections from 24-month-old WT and VavCre+Rxrabfl/fl mice; yellow arrows indicate megakaryocytes. Scale bars: 500 μm (N, upper panels), 100 μm (M, upper panels; N, bottom panels), or 50 μm (M, bottom panels). (O) Representative images of H&E-stained lungs from 24-month-old WT and VavCre+Rxrabfl/fl mice; black arrows indicate alveolar hemorrhage. Scale bars: 2.5 mm (left panels) or 250 μm (middle and right panels). Data are presented as mean ± SEM: (A-F) n = 7 to 17 mice, pooled from up to 3 independent experiments per age group and genotype; (G) n = 3 to 13 mice, pooled from up to 2 independent experiments per age group and genotype; (H) n = 4 to 24 mice, pooled from up to 3 independent experiments per age group and genotype; (I) n = 5 to 12, pooled from up to 2 independent experiments per age and genotype (spleen); (J) n = 5 to 11 mice, pooled from up to 2 independent experiments per age and genotype. Significance was determined by 2-way analysis of variance followed by the Sidak multiple comparisons test (age-paired mice) (A-J) or log-rank (Mantel-Cox) test (K), and is represented as follows: ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, and ∗∗∗∗P ≤ .0001. A, alveoli; Ad, adipocytes; AS, alveolar sac; B, bronchiole.
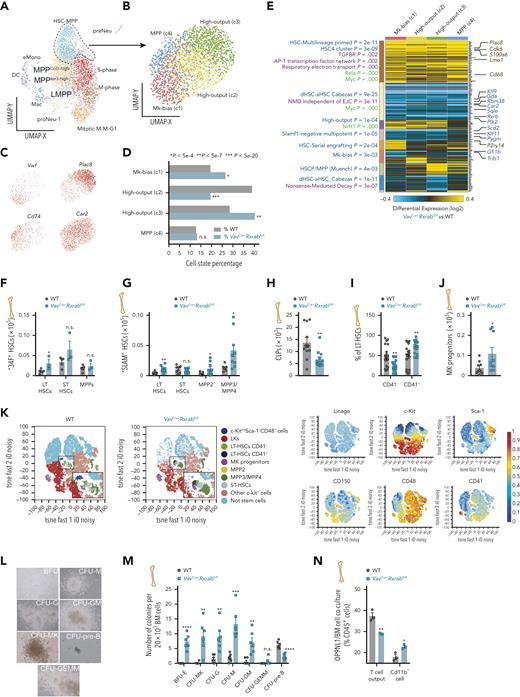
RXRα;RXRβ-deficient HSCs show myeloid-megakaryocyte skewing and impaired repopulating activity
Single-cell RNA-sequencing analysis of sorted BM LSKs identified 12 shared discrete prior-defined cell states,27 including presumptive HSC-MPP and MPP in distinct cell-cycle phases (Figure 2A; supplemental Figure 5A-B). VavCre+Rxrabfl/fl LSKs were enriched in mitotic M-M-G1, eMono, proNeu-1, and LMPP clusters, whereas WT LSKs showed enrichment in the MPPIer3-high and HSC-MPP clusters (GO-Elite Fischer Exact P ≤ 104) (supplemental Figure 5C). Subclustering of the HSC-MPP cluster detected 4 subsets, 2 of them enriched in VavCre+Rxrabfl/fl cells: (a) Vwf-enriched megakaryocyte-biased activated, cycling HSCs (c1)28 and (b) high-output cells corresponding to proliferative Plac8-expressing LT-HSCs (c3)29,30 (Figure 2B-D). In contrast, WT HSC-MPPs were enriched in Car2-positive high-output MPPs (c2)27 (Figure 2B-D). Gene set enrichment analysis of the 4 HSC-MPP subclusters identified enriched gene sets associated with HSC priming and activation5,27,31 in VavCre+Rxrabfl/fl HSC-MPPs (Figure 2E). All HSC-MPP populations from VavCre+Rxrabfl/fl mice showed upregulation of MYC-dependent mRNA transcripts (eg, Cdk6 and S100a6)32,33 and downregulation of genes associated with nonsense-mediated decay (Figure 2E).
Expansion of megakaryocyte–myeloid-biased stem cells in RXRα;RXRβ-deficient mice. (A-E) Single-cell RNA sequencing (10× Genomics) of sorted LSKs from WT and VavCre+Rxrabfl/fl BM cells (shown are clusters derived from gene set enrichment of a compendium of prior HSC/P subsets from single-cell functional studies).27 (A-B) UMAP plots of HSC/P subsets derived from VavCre+Rxrabfl/fl and WT BM LSKs (A), and of subclustering of the broader HSC-MPP cluster (B). (C) Top HSC-MPP cluster marker genes. (D) HSC-MPP population frequencies, showing significant differences between VavCre+Rxrabfl/fl and WT mice (Fischer exact P-value thresholds are indicated). (E) Heat map showing differentially expressed genes identified by cellHarmony analysis of each HSC-MPP subcluster in VavCre+Rxrabfl/fl vs WT mice. On the left of the associated clusters are shown enriched prior-defined HSC/P functional subsets (blue), pathways (purple), or curated transcription factor targets (green) (GO-Elite software); on the right of the associated clusters are shown matching bulk RNA-seq regulated genes. (F-K) Flow cytometry of BM from 5-month-old WT and VavCre+Rxrabfl/fl mice. (F-G) Absolute numbers of HSC subpopulations per femur, according to the “34F” or “SLAM” stain codes. (H) Absolute numbers of common lymphoid progenitors (CLPs) per femur. (I) Frequency of CD41+ and CD41− cells in the CD150+CD48− LT-HSC subset. (J) Absolute numbers of MK progenitor per femur. (K) Annotated t-SNE plots for the identified lineage− BM-cell populations from WT and VavCre+Rxrabfl/fl mice using the “SLAM” stain code as in G. Insets in the left panels show magnifications of the plotted HSC/P subpopulations; plots in the right panels show overlaid biexponential transformed marker expression levels (n = 3-4 per genotype). (L-M) Colony-forming unit (CFU) assay in total BM cells from 5-month-old WT and VavCre+Rxrabfl/fl mice. (L) Representative images of hematopoietic colonies identified in VavCre+Rxrabfl/fl mice after 7 days of incubation: burst-forming unit-erythroid (BFU), unipotent CFU-M (monocyte), CFU-MK (megakaryocyte), CFU-G (granulocyte), and CFU–pre-B (B lymphocyte) progenitors; bipotent CFU-GM (granulocyte, monocyte) progenitors; and multipotent CFU-GEMM (granulocyte, erythrocyte, monocyte, and megakaryocyte) progenitors. (M) Number of colonies per 20 × 103 plated BM cells (n = 6; 3 mice per genotype, with 2 technical replicates per mouse; data are representative of 2 independent experiments). (N) Lymphocyte output after 8 days of OP9NL1 cell coculture with BM cells from 5-month-old WT and VavCre+Rxrabfl/fl mice; graph shows the percentage of T cells (DN1 + DN2 + DN3 + DN4 + CD3+ cells; for gating strategy, see supplemental Figure 6F) and Cd11b+ cells within CD45+ lymphocytes. Data are shown as means ± SEM, and dots represent individual animals. Significance was determined by unpaired Student t test, and is represented as follows: ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, and ∗∗∗∗P ≤ .0001. n.s., not significant.
Expansion of megakaryocyte–myeloid-biased stem cells in RXRα;RXRβ-deficient mice. (A-E) Single-cell RNA sequencing (10× Genomics) of sorted LSKs from WT and VavCre+Rxrabfl/fl BM cells (shown are clusters derived from gene set enrichment of a compendium of prior HSC/P subsets from single-cell functional studies).27 (A-B) UMAP plots of HSC/P subsets derived from VavCre+Rxrabfl/fl and WT BM LSKs (A), and of subclustering of the broader HSC-MPP cluster (B). (C) Top HSC-MPP cluster marker genes. (D) HSC-MPP population frequencies, showing significant differences between VavCre+Rxrabfl/fl and WT mice (Fischer exact P-value thresholds are indicated). (E) Heat map showing differentially expressed genes identified by cellHarmony analysis of each HSC-MPP subcluster in VavCre+Rxrabfl/fl vs WT mice. On the left of the associated clusters are shown enriched prior-defined HSC/P functional subsets (blue), pathways (purple), or curated transcription factor targets (green) (GO-Elite software); on the right of the associated clusters are shown matching bulk RNA-seq regulated genes. (F-K) Flow cytometry of BM from 5-month-old WT and VavCre+Rxrabfl/fl mice. (F-G) Absolute numbers of HSC subpopulations per femur, according to the “34F” or “SLAM” stain codes. (H) Absolute numbers of common lymphoid progenitors (CLPs) per femur. (I) Frequency of CD41+ and CD41− cells in the CD150+CD48− LT-HSC subset. (J) Absolute numbers of MK progenitor per femur. (K) Annotated t-SNE plots for the identified lineage− BM-cell populations from WT and VavCre+Rxrabfl/fl mice using the “SLAM” stain code as in G. Insets in the left panels show magnifications of the plotted HSC/P subpopulations; plots in the right panels show overlaid biexponential transformed marker expression levels (n = 3-4 per genotype). (L-M) Colony-forming unit (CFU) assay in total BM cells from 5-month-old WT and VavCre+Rxrabfl/fl mice. (L) Representative images of hematopoietic colonies identified in VavCre+Rxrabfl/fl mice after 7 days of incubation: burst-forming unit-erythroid (BFU), unipotent CFU-M (monocyte), CFU-MK (megakaryocyte), CFU-G (granulocyte), and CFU–pre-B (B lymphocyte) progenitors; bipotent CFU-GM (granulocyte, monocyte) progenitors; and multipotent CFU-GEMM (granulocyte, erythrocyte, monocyte, and megakaryocyte) progenitors. (M) Number of colonies per 20 × 103 plated BM cells (n = 6; 3 mice per genotype, with 2 technical replicates per mouse; data are representative of 2 independent experiments). (N) Lymphocyte output after 8 days of OP9NL1 cell coculture with BM cells from 5-month-old WT and VavCre+Rxrabfl/fl mice; graph shows the percentage of T cells (DN1 + DN2 + DN3 + DN4 + CD3+ cells; for gating strategy, see supplemental Figure 6F) and Cd11b+ cells within CD45+ lymphocytes. Data are shown as means ± SEM, and dots represent individual animals. Significance was determined by unpaired Student t test, and is represented as follows: ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, and ∗∗∗∗P ≤ .0001. n.s., not significant.
Fluorescence-activated cell sorting analysis of BM LSKs according to the expression of CD34 and the FMS-like tyrosine kinase 3 (FLT3) receptor (“34F” HSCs)34 and of CD48 and CD150 (“SLAM” HSCs)22,35 revealed expansion of the most primitive HSC population (CD34−FLT3− and CD150+CD48− LT-HSCs), myeloid-biased MPPs (CD150+CD48+ MPP2),22 and CD150−CD48+ cells containing both myeloid-biased MPP322 and lymphoid-primed MPP422 (Figure 2F,G,K; supplemental Figure 6A-C). However, VavCre+Rxrabfl/fl BM showed significant depletion of downstream lymphoid-committed progenitors (Figure 2H; supplemental Figure 6D), whereas there was an expansion of CD41+ LT-HSCs (considered primed HSCs that accumulate with age36 and have short-term—and primarily myeloid—regenerative potential37) and megakaryocyte-committed progenitors38 (Figure 2I-K; supplemental Figure 5H). Consistently, functional assays of BM progenitors revealed a marked increase in the numbers of megakaryocyte/myeloid colony forming units (CFUs), a decrease of pre-B CFUs (Figure 2L-M), and a reduction in stroma-dependent T-cell output (OP9NL1/BM cocultures; Figure 2N; supplemental Figure 6F-G). Analysis of PB CFUs revealed significantly higher numbers of CFU-G (granulocyte) and CFU-GM (granulocyte/monocyte) in VavCre+Rxrabfl/fl mice (supplemental Figure 6H-I), suggesting mobilization of myeloid-biased HSCs from BM to secondary hematopoietic organs. These data demonstrate that most expanded HSC/P subsets in VavCre+Rxrabfl/fl mice were phenotypic LT-HSCs and myeloid-megakaryocyte progenitors with in vitro colony-forming ability.
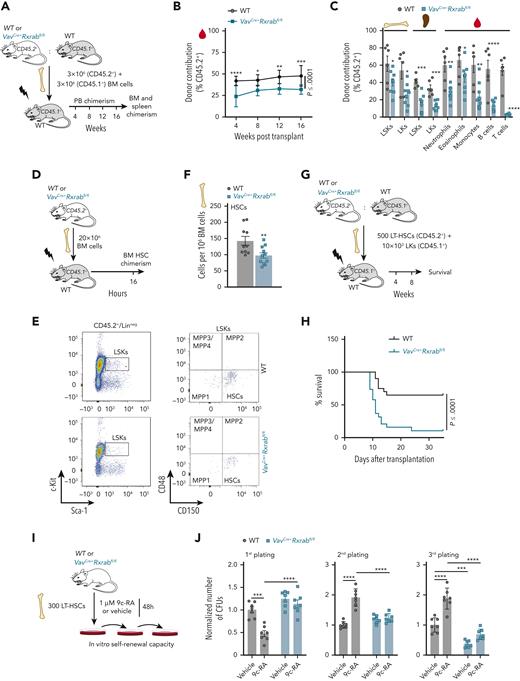
We studied the repopulation capacity of WT and VavCre+Rxrabfl/fl HSC/Ps, both alone or with a WT competitor39 (Figure 3A; supplemental Figure 7A). Analysis of PB cell chimerism revealed abnormal repopulation of VavCre+Rxrabfl/fl HSC/Ps at each time interval (Figure 3B; supplemental Figure 7B), with an especially marked decline in T-lymphocyte reconstitution (Figure 3C; supplemental Figure 7C). In addition, BM and spleen HSC/P chimerism was significantly diminished when VavCre+Rxrabfl/fl BM cells were transplanted with a WT competitor (Figure 3C). We then analyzed the homing capacity of HSCs after intravenous injection of WT or VavCre+Rxrabfl/fl BM or c-kit+ cells into lethally irradiated mice, observing a significantly reduced homing of VavCre+Rxrabfl/fl BM progenitors and HSCs (Figure 3D-F; supplemental Figure 7D-F). We analyzed the LT reconstitution capacity of HSCs by serially transferring BM cells through primary and secondary WT recipients. To avoid engraftment defects, we used Mx-1Cre+Rxrabfl/fl or WT mice as BM donors and induced Rxra/Rxrb deletion by intraperitoneal administration of pI:pC at 4 weeks after transplantation (supplemental Figure 7G-H). Although primary recipients had comparable numbers of HSC/Ps regardless of donor genotype (data not shown), secondary recipients of Mx-1Cre+Rxrabfl/fl BM had significantly fewer HSC/Ps than recipients of WT BM (supplemental Figure 7I). As a more rigorous test of HSC fitness, a limiting number of HSCs from WT or VavCre+Rxrabfl/fl mice were cotransplanted with supporting WT LKs into irradiated mice (Figure 3G). Of the irradiated animals transplanted with VavCre+Rxrabfl/fl HSCs, 90% died after the second week posttransplantation (Figure 3H), indicating that VavCre+Rxrabfl/fl HSCs were deprived of hematopoietic radioprotection activity. Accordingly, colony replating assays revealed that VavCre+Rxrabfl/fl HSCs lost their self-renewal capacity, whereas activation of RXRs with the retinoid 9c-RA enhanced their replating capacity (Figure 3I-J). These results indicate that RXRs have a cell-autonomous effect on HSC fitness, and that loss of RXRs severely compromises the capacity of HSCs to home and radioprotect.
RXRα;RXRβ-deficient HSCs are functionally defective. (A-C) Competitive transplantation assay (1:1 mix) of unfractionated BM cells from WT or VavCre+Rxrabfl/fl mice (CD45.2+) and C57BL/6 mice (CD45.1+) into lethally irradiated C57BL/6 mice (CD45.1+) (data are representative of 3 independent experiments). (A) Experimental design. (B) Fluorescence-activated cell sorting (FACS) analysis of the percentage of total donor chimerism in PB 4, 8, 12, and 16 weeks after transplantation (n = 9-10). (C) FACS analysis of cell-specific chimerism in PB, BM, and spleen 16 weeks after competitive repopulation assay. (D-F) Homing assay of total BM cells from WT or VavCre+Rxrabfl/fl mice (CD45.2+), transplanted into lethally irradiated C57BL/6 mice (CD45.1+). (D) Experimental design. (E) Representative FACS plots showing the gating strategies for analysis of CD45.2+ HSCs. (F) Total numbers of CD45.2+ HSCs found in the BM of recipient mice. (G-H) Transplantation of purified LT-HSCs from WT or VavCre+Rxrabfl/fl mice (CD45.2+) along with LKs from C57BL/6 mice (CD45.1+) into lethally irradiated C57BL/6 mice (CD45.1+) (n = 19-20; data pooled from 2 independent experiments). (G) Experimental design. (H) Kaplan-Meier survival plot. (I-J) Serial CFU plating assay after 48-hours in vitro treatment of purified LT-HSCs from 5-month-old WT or VavCre+Rxrabfl/fl mice with 9c-RA or vehicle. (I) Experimental design. (J) Number of CFUs normalized to control vehicle treatment of each corresponding plating (data pooled from 2 independent experiments). All data are shown as means ± SEM, and dots represent individual animals. Significance was determined by 2-way analysis of variance (ANOVA) followed by the Sidak multiple comparisons test (B), unpaired Student t test (C and F), log-rank (Mantel-Cox) test (H), or ordinary 1-way ANOVA (J), and is represented as follows: ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, and ∗∗∗∗P ≤ .0001.
RXRα;RXRβ-deficient HSCs are functionally defective. (A-C) Competitive transplantation assay (1:1 mix) of unfractionated BM cells from WT or VavCre+Rxrabfl/fl mice (CD45.2+) and C57BL/6 mice (CD45.1+) into lethally irradiated C57BL/6 mice (CD45.1+) (data are representative of 3 independent experiments). (A) Experimental design. (B) Fluorescence-activated cell sorting (FACS) analysis of the percentage of total donor chimerism in PB 4, 8, 12, and 16 weeks after transplantation (n = 9-10). (C) FACS analysis of cell-specific chimerism in PB, BM, and spleen 16 weeks after competitive repopulation assay. (D-F) Homing assay of total BM cells from WT or VavCre+Rxrabfl/fl mice (CD45.2+), transplanted into lethally irradiated C57BL/6 mice (CD45.1+). (D) Experimental design. (E) Representative FACS plots showing the gating strategies for analysis of CD45.2+ HSCs. (F) Total numbers of CD45.2+ HSCs found in the BM of recipient mice. (G-H) Transplantation of purified LT-HSCs from WT or VavCre+Rxrabfl/fl mice (CD45.2+) along with LKs from C57BL/6 mice (CD45.1+) into lethally irradiated C57BL/6 mice (CD45.1+) (n = 19-20; data pooled from 2 independent experiments). (G) Experimental design. (H) Kaplan-Meier survival plot. (I-J) Serial CFU plating assay after 48-hours in vitro treatment of purified LT-HSCs from 5-month-old WT or VavCre+Rxrabfl/fl mice with 9c-RA or vehicle. (I) Experimental design. (J) Number of CFUs normalized to control vehicle treatment of each corresponding plating (data pooled from 2 independent experiments). All data are shown as means ± SEM, and dots represent individual animals. Significance was determined by 2-way analysis of variance (ANOVA) followed by the Sidak multiple comparisons test (B), unpaired Student t test (C and F), log-rank (Mantel-Cox) test (H), or ordinary 1-way ANOVA (J), and is represented as follows: ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, and ∗∗∗∗P ≤ .0001.
Quiescent HSCs transition to an active proliferative state in the absence of RXRs
To understand the role of RXRs in HSC/P proliferation, we performed a baseline in vivo 5-bromo-2′-deoxyuridine (BrdU) pulse, demonstrating that VavCre+Rxrabfl/fl LSKs and LT-HSCs progress faster than their WT counterparts through the cell-cycle S phase (Figure 4A). Pyronin Y coupled to Hoechst 33342 labeling indicated that VavCre+Rxrabfl/fl LSKs and LT-HSCs had higher cell-cycle activity and fewer G0 phase cells than their WT counterparts, indicating loss of quiescence by RXRα;RXRβ-deficient HSC/Ps (Figure 4B). In addition, annexin-V/4′,6-diamidino-2-phenylindole (DAPI) labeling assays showed reduced frequencies of VavCre+Rxrabfl/fl LSKs and LKs entering apoptosis (supplemental Figure 7J-K).
Dual lack of RXRα and RXRβ in HSCs leads to cell-cycle activation and quiescence exit. (A) Representative flow cytometry density plots showing 5-bromo-2′-deoxyuridine (BrdU) incorporation by LSKs and CD150+CD48− LT-HSCs after a 24-hour pulse (left); the plot (right) shows the frequencies of cells with BrdU incorporation (data are pooled from 2 independent experiments). (B) Representative flow cytometry density plots showing pyronin Y and Hoechst 33342 staining by LSKs and CD150+CD48− LT-HSCs (upper plots); the lower plots show frequencies of cells with double pyronin Y and Hoechst 33342 staining (data are pooled from 2 independent experiments). (C-E) Recovery of hematopoietic cells after 5-fluorouracil (5-FU) administration to VavCre+Rxrabfl/fl and WT mice. (C) Experimental design. (D) Time course of PB-cell counts (baseline was defined as the mean reading on day 0, expressed as 100%; n = 9-11 mice per genotype). (E) Absolute HSC/P numbers in total BM 20 days after 5-FU administration. (F-H) HSC/P proliferation 5 days after 5-FU administration to VavCre+Rxrabfl/fl and WT mice. (F) Experimental design. (G) Representative flow cytometry density plots showing BrdU incorporation by LSKs and CD150+CD48− LT-HSCs. (H) Frequencies of BrdU-positive cells. All data are presented as means ± SEM, and dots represent individual animals. Significance was determined by unpaired Student t test (A, B, E, and H) or 2-way analysis of variance followed by the Sidak multiple comparisons test (D; age-paired mice), and is represented as follows: ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, and ∗∗∗∗P ≤ .0001. n.s., not significant.
Dual lack of RXRα and RXRβ in HSCs leads to cell-cycle activation and quiescence exit. (A) Representative flow cytometry density plots showing 5-bromo-2′-deoxyuridine (BrdU) incorporation by LSKs and CD150+CD48− LT-HSCs after a 24-hour pulse (left); the plot (right) shows the frequencies of cells with BrdU incorporation (data are pooled from 2 independent experiments). (B) Representative flow cytometry density plots showing pyronin Y and Hoechst 33342 staining by LSKs and CD150+CD48− LT-HSCs (upper plots); the lower plots show frequencies of cells with double pyronin Y and Hoechst 33342 staining (data are pooled from 2 independent experiments). (C-E) Recovery of hematopoietic cells after 5-fluorouracil (5-FU) administration to VavCre+Rxrabfl/fl and WT mice. (C) Experimental design. (D) Time course of PB-cell counts (baseline was defined as the mean reading on day 0, expressed as 100%; n = 9-11 mice per genotype). (E) Absolute HSC/P numbers in total BM 20 days after 5-FU administration. (F-H) HSC/P proliferation 5 days after 5-FU administration to VavCre+Rxrabfl/fl and WT mice. (F) Experimental design. (G) Representative flow cytometry density plots showing BrdU incorporation by LSKs and CD150+CD48− LT-HSCs. (H) Frequencies of BrdU-positive cells. All data are presented as means ± SEM, and dots represent individual animals. Significance was determined by unpaired Student t test (A, B, E, and H) or 2-way analysis of variance followed by the Sidak multiple comparisons test (D; age-paired mice), and is represented as follows: ∗P ≤ .05, ∗∗P ≤ .01, ∗∗∗P ≤ .001, and ∗∗∗∗P ≤ .0001. n.s., not significant.
We treated VavCre+Rxrabfl/fl and WT mice with 5-fluorouracil (5-FU) and tracked stressed hematopoietic recovery over 20 days (Figure 4C). Early (day 10-18) neutrophil and platelet recoveries were significantly delayed in VavCre+Rxrabfl/fl mice (Figure 4D), confirming a fitness deficit in the response of RXRα;RXRβ-deficient HSCs. However, VavCre+Rxrabfl/fl myeloid cells showed higher proliferative capacity in the late recovery phase, as shown by a significantly higher rebound than WT on posttreatment days 18 to 20 (2.15 ± 0.75 vs 1.26 ± 0.40 fold change; P = .0055), with this effect especially pronounced for monocytes (4.24 ± 2.61 vs 1.14 ± 0.87 fold change; P = .0034) (Figure 4D). The frequencies of BM LT-HSCs and MPPs were higher in VavCre+Rxrabfl/fl mice on posttreatment day 20 (Figure 4E), and BrdU labeling revealed higher proliferation rates of VavCre+Rxrabfl/fl LSKs and LT-HSCs on post–5-FU treatment day 5 (Figure 4F-G). These results indicate that RXRα;RXRβ expression is required for the maintenance of HSC quiescence and death rate in steady-state and stress hematopoiesis.
RXRα;RXRβ-deficient HSCs have an aged-like gene signature and show MYC signaling pathway activation and alternative splicing with intron retention
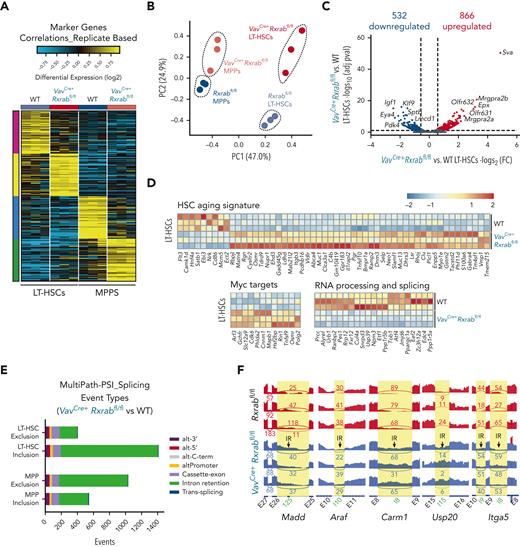
We performed a bulk RNA-seq analysis on LT-HSCs and MPPs from 5-month-old WT and VavCre+Rxrabfl/fl mice. Analysis of the predominant gene expression patterns identified uniquely expressed transcripts in each of the separate gates (Figure 5A). Although gene expression differed between VavCre+Rxrabfl/fl LT-HSCs and MPPs and their WT counterparts, the greatest between-sample differences were by cell state rather than genotype. This observation was further supported by principal component (PC) analysis of detectable genes, which showed that the major source of variance (PC1, 47%) was cell state, with PC2 corresponding to genotype (Figure 5B). VavCre+Rxrabfl/fl LT-HSCs were characterized by predominant upregulation of gene expression (fold change [FC] ≥ 1.5 or ≤ −1.5; adjusted P ≤ 0.1) (Figure 5C; supplemental Tables 2 and 3). In contrast, VavCre+Rxrabfl/fl MPPs showed prominent transcript downregulation vs WT MPPs (FC ≥ 1.5 or ≤ −1.5; adjusted P ≤ 0.1) (supplemental Figure 8A; supplemental Tables 4 and 5). Top downregulated genes in VavCre+Rxrabfl/fl LT-HSCs included molecules important for lymphoid and erythroid development and function (Klf9 and Sptb)40,41 and Pdk4, which is essential for LT-HSC cell-cycle quiescence and metabolism42 (Figure 5C; supplemental Tables 2 and 3).
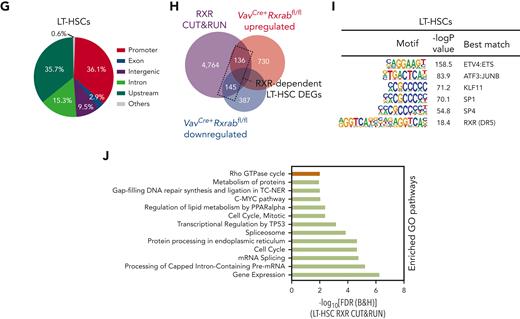
Lack of RXRα and RXRβ provokes an HSC-aged signature, MYC signaling pathway activation, and intron retention in LT-HSCs. (A-F) Bulk transcriptome of WT and VavCre+Rxrabfl/fl LT-HSCs and MPP BM cells. (A) Heat map showing the top specific marker genes per sorted population/genotype, obtained with MarkerFinder software. (B) Principal component (PC) analysis of the transcriptomes in A. (C) Volcano plot showing the global transcriptional changes in VavCre+Rxrabfl/fl vs WT LT-HSCs determined by bulk RNA-seq. Each circle represents one differentially expressed gene (DEG), and colored circles represent DEGs showing significant upregulation (adjusted P [P-adj] ≤ .1 and FC ≥ 1.5 [red]) or significant downregulation (P-adj ≤ .1 and FC ≤ −1.5 [blue]). Normalized expression values from bulk RNA-seq data are provided in supplemental Table 2. (D) Heat map showing normalized log2FC for the expression of HSC aging signature genes, MYC pathway genes, and RNA processing and splicing-related genes in WT and VavCre+Rxrabfl/fl LT-HSCs. (E-F) Number of alternative splicing events in VavCre+Rxrabfl/fl vs WT LT-HSCs and MPPs for distinct alternative exon and intron usage forms. Splicing events associated with exon or intron inclusion (increased relative expression) vs exclusion are shown separately for LT-HSC and MPP comparisons. (F) SashimiPlot read-level visualization of intron–retention-associated splicing events in WT (red) and VavCre+Rxrabfl/fl (blue) bulk RNA-seq LT-HSC samples. Exon-exon junction-spanning reads are denoted with curved lines, together with associated read counts. (G-J) RXRα whole genome binding (CUT&RUNseq). (G) Genomic distribution of enriched regions in WT LT-HSCs identified in the RXR CUT&RUNseq data set. (H) Overlaps of total LT-HSC RXRα CUT&RUNseq peaks with bulk RNA-seq upregulated and dowregulated genes in VavCre+Rxrabfl/fl vs WT LT-HSCs. (I) HOMER analysis of known motifs in WT LT-HSC RXRα peaks. (J) List of significantly enriched Gene Ontology (GO) terms in WT LT-HSC promoters (±1 kb from the transcription start site [TSS]; green bars) and upstream regions (±1-20 kb from the TSS; brown bar) for diverse pathways on analysis of the RXRα CUT&RUNseq data set using the Cincinnati Children’s Hospital Medical Center (CCHMC) ToppGene suite (https://toppgene.cchmc.org/enrichment.jsp) (false discovery rate [Benjamini and Hochberg (B&H)] ≤ 0.01).
Lack of RXRα and RXRβ provokes an HSC-aged signature, MYC signaling pathway activation, and intron retention in LT-HSCs. (A-F) Bulk transcriptome of WT and VavCre+Rxrabfl/fl LT-HSCs and MPP BM cells. (A) Heat map showing the top specific marker genes per sorted population/genotype, obtained with MarkerFinder software. (B) Principal component (PC) analysis of the transcriptomes in A. (C) Volcano plot showing the global transcriptional changes in VavCre+Rxrabfl/fl vs WT LT-HSCs determined by bulk RNA-seq. Each circle represents one differentially expressed gene (DEG), and colored circles represent DEGs showing significant upregulation (adjusted P [P-adj] ≤ .1 and FC ≥ 1.5 [red]) or significant downregulation (P-adj ≤ .1 and FC ≤ −1.5 [blue]). Normalized expression values from bulk RNA-seq data are provided in supplemental Table 2. (D) Heat map showing normalized log2FC for the expression of HSC aging signature genes, MYC pathway genes, and RNA processing and splicing-related genes in WT and VavCre+Rxrabfl/fl LT-HSCs. (E-F) Number of alternative splicing events in VavCre+Rxrabfl/fl vs WT LT-HSCs and MPPs for distinct alternative exon and intron usage forms. Splicing events associated with exon or intron inclusion (increased relative expression) vs exclusion are shown separately for LT-HSC and MPP comparisons. (F) SashimiPlot read-level visualization of intron–retention-associated splicing events in WT (red) and VavCre+Rxrabfl/fl (blue) bulk RNA-seq LT-HSC samples. Exon-exon junction-spanning reads are denoted with curved lines, together with associated read counts. (G-J) RXRα whole genome binding (CUT&RUNseq). (G) Genomic distribution of enriched regions in WT LT-HSCs identified in the RXR CUT&RUNseq data set. (H) Overlaps of total LT-HSC RXRα CUT&RUNseq peaks with bulk RNA-seq upregulated and dowregulated genes in VavCre+Rxrabfl/fl vs WT LT-HSCs. (I) HOMER analysis of known motifs in WT LT-HSC RXRα peaks. (J) List of significantly enriched Gene Ontology (GO) terms in WT LT-HSC promoters (±1 kb from the transcription start site [TSS]; green bars) and upstream regions (±1-20 kb from the TSS; brown bar) for diverse pathways on analysis of the RXRα CUT&RUNseq data set using the Cincinnati Children’s Hospital Medical Center (CCHMC) ToppGene suite (https://toppgene.cchmc.org/enrichment.jsp) (false discovery rate [Benjamini and Hochberg (B&H)] ≤ 0.01).
Gene ontology enrichment analysis revealed that downregulated pathways in VavCre+Rxrabfl/fl LT-HSCs were associated with splicing regulation and erythrocyte development, whereas enriched pathways included cell-cell adhesion and leukocyte function (supplemental Figure 8B-C). In VavCre+Rxrabfl/fl MPPs, upregulated pathways were related to oxidative stress, whereas downregulated pathways included tumor necrosis factor-α and interleukin-6 production and T-cell activation (supplemental Figure 8B-C). Differentially expressed genes (DEGs) in VavCre+Rxrabfl/fl LT-HSCs included genes previously reported to be deregulated in aged HSCs43 (Figure 5D). Other DEGs in VavCre+Rxrabfl/fl LT-HSCs included MYC targets44 and RNA processing and splicing regulators (Figure 5D). To determine whether RNA splicing alterations might be involved in the aged-HSC signature of VavCre+Rxrabfl/fl LT-HSCs, we evaluated alternative splicing and intron retention.31,45,46 This analysis identified 1841 unique known and novel alternative splicing events differentiating WT from VavCre+Rxrabfl/fl LT-HSCs, and 1638 events differentiating WT from VavCre+Rxrabfl/fl MPPs (empirical Bayes moderated t-test P < .05) (Figure 5E). By considering inclusion (exon/intron gain) and exclusion (exon/intron loss) separately, we found that most events involved differential intron retention, preferentially in VavCre+Rxrabfl/fl LT-HSCs (Figure 5E). Alternative splicing specific to VavCre+Rxrabfl/fl LT-HSCs was associated with programmed cell death (eg, Madd), cell-cycle arrest (eg, Araf), chromatin modification (eg, Araf), NR-mediated signaling (eg, Carm1), thiol-ester hydrolase activity (eg, Usp20), and cell-cell communication (eg, Itga5); these findings were verified by genomic read visualization (Figure 5F). Significantly regulated splicing inclusion events in VavCre+Rxrabfl/fl LT-HSCs were enriched in genes linked to GTPase activity and cell death, whereas significantly regulated exclusion splicing events were enriched in genes involved in the cell cycle, autophagy, and stress response control (Gene Ontology) (supplemental Figure 8D).
To generate a genome-wide profile of RXR binding in LT-HSCs and MPPs, we performed RXRα CUT&RUNseq assays. This analysis identified >6352 binding sites in LT-HSCs and >21 046 RXR binding sites in MPPs, with sites preferentially located in promoter and upstream regions (Figure 5G; supplemental Figure 8E; supplemental Tables 6 and 7). Overlap analysis with bulk RNA-seq data demonstrated that a high number of DEGs in VavCre+Rxrabfl/fl cells were directly regulated by RXRs (Figure 5H; supplemental Figure 8F). Motif analysis of LT-HSC RXR peaks indicated enrichment for RXR binding sites and for TFs essential for the maintenance and differentiation of HSCs, including erythroblast transformation specific (ETS) translocation variant 4 (ETV4),47 specificity protein 1 (SP1),48 and cyclic AMP-dependent transcription factor (ATF3)49 (Figure 5I). Similar data were obtained in MPPs (supplemental Figure 8G). Functional annotation clustering analysis of the RXR cistrome identified a variety of enriched pathways in LT-HSCs and MPPs, including cell cycle and the MYC pathway, as well as processes involved in mRNA processing and splicing (Figure 5J; supplemental Figure 8H). Our results provide evidence that RXRs function as master TFs that maintain a young-like HSC transcriptional signature, low MYC-dependent transcriptional activity, and appropriate splicing in LT-HSCs.
RXR deficiency leads to chromatin openness at promoter and enhancer regions in LT-HSCs
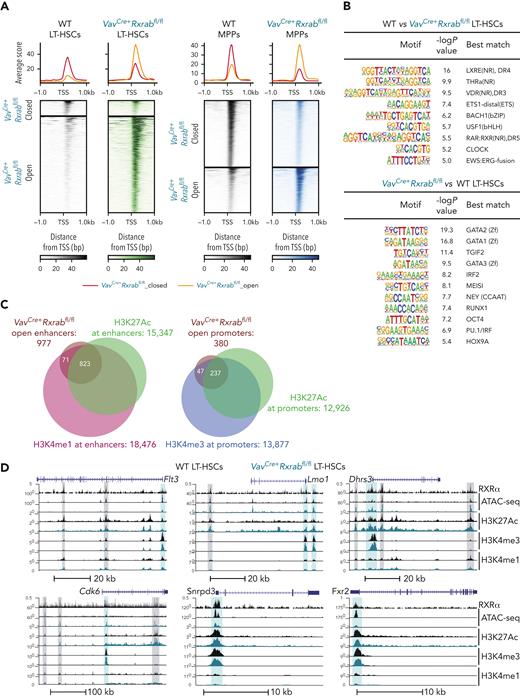
Assay for transposase-accessible chromatin with sequencing (ATAC-seq) revealed a strong spike in chromatin-accessible peaks in VavCre+Rxrabfl/fl vs WT LT-HSCs (z score ≤ −2 or ≥ 2; P ≤ .05) (Figure 6A; supplemental Tables 8 and 9), indicating that RXRα;RXRβ deficiency leads to chromatin openness in LT-HSCs. In contrast, VavCre+Rxrabfl/fl MPPs had a higher proportion of closed peaks than WT MPPs (FC ≤ −2 or ≥ 2; false discovery rate ≤ 0.05) (Figure 6A; supplemental Tables 10 and 11). Analysis of peak distribution demonstrated a reorganization of the chromatin landscape in VavCre+Rxrabfl/fl LT-HSCs but not MPPs (supplemental Figure 9A-B). Motif enrichment analysis showed a reduction in peaks enriched in binding sites for NRs in VavCre+Rxrabfl/fl LT-HSCs and MPPs (P ≤ .01; Figure 6B; supplemental Figure 9C). Conversely, VavCre+Rxrabfl/fl LT-HSCs were highly enriched in binding sites for several TFs with roles in hematopoiesis, including the master hematopoiesis regulators GATA (consensus DNA sequence [T/A]GATA[A/G])-binding factors GATA1, GATA2, and GATA350 and RUNT-related transcription factor 1 (RUNX1)51; myeloid ecotropic viral integration site 1 (MEIS1), which is overexpressed in certain leukemias52; the myeloid differentiation master TF PU.153; and the HSC-activity marker homeobox protein Hox-A9 (HOX9A)54 (Figure 6B). Similar binding-site enrichment was found in VavCre+Rxrabfl/fl MPPs (supplemental Figure 9C). Overlap analysis between RXR-binding sites and VavCre+Rxrabfl/fl vs WT differentially accessible regions (DARs) revealed that around 50% of changes in MPP chromatin accessibility were RXR dependent, whereas a lower proportion of DARs colocalized with RXR-binding sites in LT-HSCs (supplemental Figure 9D-E).
Chromatin openness at enhancer and promoter sites in RXRα;RXRβ-deficient LT-HSCs. (A) Heat map of assay for transposase-accessible chromatin with sequencing (ATAC-seq) signals located in transcription start site (TSS) flanking regions (±1 kb) in 5-month-old VavCre+Rxrabfl/fl and WT LT-HSCs and MPPs. (B) HOMER known motif analysis of ATAC-seq peaks. The top panels shows transcription factor motifs enriched in WT LT-HSCs relative to the background of VavCre+Rxrabfl/fl LT-HSC peaks. The bottom panel shows transcription factor motifs enriched in VavCre+Rxrabfl/fl LT-HSCs relative to the background of WT LT-HSC peaks. (C) Peak annotations identified in an overlap analysis between the open promoter and enhancer regions in LT-HSCs identified in the ATAC-seq experiment (defined by genomic distribution; see also supplemental Figure 8A), and regions marked by high H3K4me3 and H3K27Ac, and ±1 kb from TSS (putative promoters), high H3K4me1 and H3K27Ac, and >1 kb and <−1 kb from the TSS (putative active enhancers), and high H3K4me1 but no H3K27Ac, and >1 kb and <−1 kb from the TSS (putative poised enhancers). (D) University of California, Santa Cruz (UCSC) genome browser track examples of active enhancers (vertical gray highlights) and promoters (vertical blue highlights) within loci that overlap RXRα CUT&RUNseq and ATAC-seq peaks.
Chromatin openness at enhancer and promoter sites in RXRα;RXRβ-deficient LT-HSCs. (A) Heat map of assay for transposase-accessible chromatin with sequencing (ATAC-seq) signals located in transcription start site (TSS) flanking regions (±1 kb) in 5-month-old VavCre+Rxrabfl/fl and WT LT-HSCs and MPPs. (B) HOMER known motif analysis of ATAC-seq peaks. The top panels shows transcription factor motifs enriched in WT LT-HSCs relative to the background of VavCre+Rxrabfl/fl LT-HSC peaks. The bottom panel shows transcription factor motifs enriched in VavCre+Rxrabfl/fl LT-HSCs relative to the background of WT LT-HSC peaks. (C) Peak annotations identified in an overlap analysis between the open promoter and enhancer regions in LT-HSCs identified in the ATAC-seq experiment (defined by genomic distribution; see also supplemental Figure 8A), and regions marked by high H3K4me3 and H3K27Ac, and ±1 kb from TSS (putative promoters), high H3K4me1 and H3K27Ac, and >1 kb and <−1 kb from the TSS (putative active enhancers), and high H3K4me1 but no H3K27Ac, and >1 kb and <−1 kb from the TSS (putative poised enhancers). (D) University of California, Santa Cruz (UCSC) genome browser track examples of active enhancers (vertical gray highlights) and promoters (vertical blue highlights) within loci that overlap RXRα CUT&RUNseq and ATAC-seq peaks.
We performed CUT&RUNseq assays to determine the monomethylation and trimethylation status of lysine 4 (H3K4me1 and H3K4me3) and the acetylation status of lysine 27 (H3K27ac) in histone 3 (supplemental Figure 9F). An overlap analysis between open DARs and regions marked by (1) high H3K4me3 and H3K27Ac (located ±1 kb from a transcription start site [TSS]; putative promoters), (2) high H3K4me1 and H3K27Ac (located >1 kb and <−1 kb from a TSS; putative active enhancers), and (3) high H3K4me1 but no H3K27Ac (located >1 kb and <−1 kb from a TSS; putative poised enhancers) revealed that most open DARs in VavCre+Rxrabfl/fl LT-HSCs coincided with active promoters and enhancers (Figure 6C). Double H3K4me3;H3K27Ac- and/or H3K4me1;H3K27Ac-enriched regions overlapping with RXR-binding sites and DARs were found in loci harboring HSC-aging signature genes,43 leukemia oncogenes, MYC targets, and RNA processing genes, including Flt3, Lmo1, Dhrs3, Cdk6, Snrpd3, and Fxr2 (Figure 6D). These data indicate that RXRs contribute to the LT-HSC maintenance of chromatin condensation and transcriptional repression, which are hallmarks of cell quiescence.55
Myc haploinsufficiency rescues regeneration of phenotypically identifiable LT-HSCs in RXR-deficient mice
To determine if MYC mediates the role of RXRs in HSC fitness, we crossed Myc-haploinsufficient mice56 with VavCre+Rxrabfl/fl mice, generating VavCre+Rxrabfl/fl-Myc+/fl mice. The functional capacities of equal numbers of BM HSC/Ps from WT, VavCre+Rxrabfl/fl, or VavCre+Rxrabfl/fl-Myc+/fl mice were tested in competitive repopulation assays (Figure 7A; supplemental Figure 10A). Although the PB donor contribution of VavCre+Rxrabfl/fl-Myc+/fl mice was even lower than that of VavCre+Rxrabfl/fl mice (supplemental Figure 10B), Myc haploinsufficiency rescued the regeneration of LT-HSCs (but not other HSC/Ps) (Figure 7B; supplemental Figure 10C). Cell-cycle analysis showed that Myc haploinsufficiency restored the level of VavCre+Rxrabfl/fl LT-HSCs in G0 phase and the percentage of proliferating cells back to WT levels (Figure 7C-D). We performed a pair-daughter first HSC division assay, in which MYC upregulation in daughter cells is a hallmark of loss of self-renewing divisions.57,58 Lack of RXRs entailed an approximately twofold increase in the frequency of differentiated/committed daughter cells, concomitant with a reduction in symmetric self-renewal division modes (Figure 7E-F). These changes in fate division were restored to WT levels in VavCre+Rxrabfl/fl-Myc+/fl mice (Figure 7E-F). The rescue of self-renewing cells was also observed in vitro by serial plating analysis (Figure 7G). However, Myc haploinsufficiency did not restore the pre–B-cell progenitor content in BM (supplemental Figure 10D).
Myc haploinsufficiency rescues regeneration, transcriptome, and spliceosome of phenotypically identifiable LT-HSCs. (A-B) Competitive transplantation assay of unfractionated BM cells from 4-month-old WT, VavCre+Rxrabfl/fl, and VavCre+Rxrabfl/fl-Myc+/fl mice (CD45.2+) in a 1:1 ratio with C57BL/6 BM cells (CD45.1+) into lethally irradiated C57BL/6 mice (CD45.1+) (data pooled from 2 independent experiments). (A) Experimental scheme. (B) Percentages of CD45.2+ BM LT-HSCs at 16 weeks after transplantation. (C) Frequencies of CD150+CD48− LT-HSCs from WT, VavCre+Rxrabfl/fl, and VavCre+Rxrabfl/fl-Myc+/fl mice in the G0 phase of the cell cycle (pyronin Y−/Hoechst 33342−; data pooled from 3 independent experiments). (D) Percentage of 5-bromo-2′-deoxyuridine–positive (BrdU+) CD150+CD48− LT-HSCs from WT, VavCre+Rxrabfl/fl, and VavCre+Rxrabfl/fl-Myc+/fl mice after 1-hour pulse with BrdU. (E-F) Immunofluorescence analysis, showing different division modes according to MYC expression in paired daughter LT-HSCs from WT, VavCre+Rxrabfl/fl, and VavCre+Rxrabfl/fl-Myc+/fl mice. (E) Representative images showing (1) symmetric self-renewal (absence of MYC expression in paired daughter cells), (2) asymmetric division (high vs low MYC expression in paired daughter cells), and (3) symmetric differentiation/commitment (MYC expression in both cells); scale bar, 10 μm. (F) Percentage of LT-HSCs corresponding to division modes 1, 2, and 3 as in E; n = 60 division pairs captured from 5 WT mice; n = 48 division pairs captured from 3 VavCre+Rxrabfl/fl mice; and n = 37 division pairs captured from 5 VavCre+Rxrabfl/fl-Myc+/fl mice. Analyses were performed in 2 independent experiments. (G) Serial CFU plating assay in total BM cells from WT, VavCre+Rxrabfl/fl, or VavCre+Rxrabfl/fl-Myc+/fl mice. (H-L) Bulk transcriptome of WT, VavCre+Rxrabfl/fl, and VavCre+Rxrabfl/fl-Myc+/fl LT-HSCs. Normalized expression values from bulk RNA-seq data are provided in supplemental Tables 12-15. (H) Rescued differentially expressed genes in VavCre+Rxrabfl/fl-Myc+/fl vs VavCre+Rxrabfl/fl LT-HSCs (eBayes t-test P < .05; fold > 1.2), compared with VavCre+Rxrabfl/fl-Myc+/fl vs WT LT-HSCs (eBayes t-test P < .05; false discovery rate corrected; fold > 1.5). (I) Heat map of the 435 rescued genes showing silencing of VavCre+Rxrabfl/fl LT-HSC–induced transcripts by Myc+/fl expression. On the left are shown example rescued genes (Figure 5D; TFs or RNA-binding regulators). (J) Rescued alternative splicing events in VavCre+Rxrabfl/fl-Myc+/fl vs VavCre+Rxrabfl/fl LT-HSCs, compared with VavCre+Rxrabfl/fl-Myc+/fl vs WT LT-HSCs (eBayes t-test P < .05; δ percentage spliced in [PSI] > 0.1 for both comparisons). (K) Heat map of 154 unique splicing events, organized by increased junction PSI value in VavCre+Rxrabfl/fl vs WT LT-HSCs, with exemplar intron retention events (green tick mark) and genes symbols called out. (L) Number of observed unique annotated splicing events in the 154 rescued splicing events, by exon/intron inclusion or exclusion. Splicing events associated with exon or intron inclusion (increased relative expression) vs exclusion are shown separately. Results are presented as means ± SEM, and dots represent individual animals. Significance was determined by ordinary 1-way analysis of variance, and is represented as follows: ∗P ≤ .05, ∗∗P ≤ .01, and ∗∗∗P ≤ .001. DAPI, 4′,6-diamidino-2-phenylindole; n.s., not significant.
Myc haploinsufficiency rescues regeneration, transcriptome, and spliceosome of phenotypically identifiable LT-HSCs. (A-B) Competitive transplantation assay of unfractionated BM cells from 4-month-old WT, VavCre+Rxrabfl/fl, and VavCre+Rxrabfl/fl-Myc+/fl mice (CD45.2+) in a 1:1 ratio with C57BL/6 BM cells (CD45.1+) into lethally irradiated C57BL/6 mice (CD45.1+) (data pooled from 2 independent experiments). (A) Experimental scheme. (B) Percentages of CD45.2+ BM LT-HSCs at 16 weeks after transplantation. (C) Frequencies of CD150+CD48− LT-HSCs from WT, VavCre+Rxrabfl/fl, and VavCre+Rxrabfl/fl-Myc+/fl mice in the G0 phase of the cell cycle (pyronin Y−/Hoechst 33342−; data pooled from 3 independent experiments). (D) Percentage of 5-bromo-2′-deoxyuridine–positive (BrdU+) CD150+CD48− LT-HSCs from WT, VavCre+Rxrabfl/fl, and VavCre+Rxrabfl/fl-Myc+/fl mice after 1-hour pulse with BrdU. (E-F) Immunofluorescence analysis, showing different division modes according to MYC expression in paired daughter LT-HSCs from WT, VavCre+Rxrabfl/fl, and VavCre+Rxrabfl/fl-Myc+/fl mice. (E) Representative images showing (1) symmetric self-renewal (absence of MYC expression in paired daughter cells), (2) asymmetric division (high vs low MYC expression in paired daughter cells), and (3) symmetric differentiation/commitment (MYC expression in both cells); scale bar, 10 μm. (F) Percentage of LT-HSCs corresponding to division modes 1, 2, and 3 as in E; n = 60 division pairs captured from 5 WT mice; n = 48 division pairs captured from 3 VavCre+Rxrabfl/fl mice; and n = 37 division pairs captured from 5 VavCre+Rxrabfl/fl-Myc+/fl mice. Analyses were performed in 2 independent experiments. (G) Serial CFU plating assay in total BM cells from WT, VavCre+Rxrabfl/fl, or VavCre+Rxrabfl/fl-Myc+/fl mice. (H-L) Bulk transcriptome of WT, VavCre+Rxrabfl/fl, and VavCre+Rxrabfl/fl-Myc+/fl LT-HSCs. Normalized expression values from bulk RNA-seq data are provided in supplemental Tables 12-15. (H) Rescued differentially expressed genes in VavCre+Rxrabfl/fl-Myc+/fl vs VavCre+Rxrabfl/fl LT-HSCs (eBayes t-test P < .05; fold > 1.2), compared with VavCre+Rxrabfl/fl-Myc+/fl vs WT LT-HSCs (eBayes t-test P < .05; false discovery rate corrected; fold > 1.5). (I) Heat map of the 435 rescued genes showing silencing of VavCre+Rxrabfl/fl LT-HSC–induced transcripts by Myc+/fl expression. On the left are shown example rescued genes (Figure 5D; TFs or RNA-binding regulators). (J) Rescued alternative splicing events in VavCre+Rxrabfl/fl-Myc+/fl vs VavCre+Rxrabfl/fl LT-HSCs, compared with VavCre+Rxrabfl/fl-Myc+/fl vs WT LT-HSCs (eBayes t-test P < .05; δ percentage spliced in [PSI] > 0.1 for both comparisons). (K) Heat map of 154 unique splicing events, organized by increased junction PSI value in VavCre+Rxrabfl/fl vs WT LT-HSCs, with exemplar intron retention events (green tick mark) and genes symbols called out. (L) Number of observed unique annotated splicing events in the 154 rescued splicing events, by exon/intron inclusion or exclusion. Splicing events associated with exon or intron inclusion (increased relative expression) vs exclusion are shown separately. Results are presented as means ± SEM, and dots represent individual animals. Significance was determined by ordinary 1-way analysis of variance, and is represented as follows: ∗P ≤ .05, ∗∗P ≤ .01, and ∗∗∗P ≤ .001. DAPI, 4′,6-diamidino-2-phenylindole; n.s., not significant.
Next, we performed bulk RNA-seq in WT, VavCre+Rxrabfl/fl, and VavCre+Rxrabfl/fl-Myc+/fl LT-HSCs. Analyses of these data show that Myc haploinsufficiency partially restores VavCre+Rxrabfl/fl transcriptional (40%) and splicing (13%) defects (Figure 7H-L; supplemental Figure 10E; supplemental Tables 12-15). Rescued DEGs included diverse transcriptional regulators (eg, Gfi1 and Irf7) and few RNA-binding proteins (eg, RnaseI and Snapc4) (Figure 7I). Although only a small percentage of RXR-dependent splicing events were rescued (Figure 7J-L), 62% of them were enriched in the reversal of intron retention in genes involved in posttranscriptional regulation (eg, Sf1 and U2af2), regulation of translation (eg, Tcea2 and Ago2), chromatin DNA binding (eg, Tox and Per1), and transcriptional regulation (eg, Sp100 and Setd6) (Figure 7K). Our results demonstrate that MYC activity is the key RXR-dependent molecular regulator of LT-HSC fitness, in vivo regeneration activity, as well as transcriptome and spliceosome.
Discussion
In this study, we demonstrate that RXRs are essential for the maintenance of HSC activity and balanced hematopoietic differentiation. Dual lack of RXRα and RXRβ in HSCs leads to chromatin openness and the acquisition of a premature aged-like transcriptional and phenotypic signature in LT-HSCs. As a result, these cells transition from a dormant to a proliferative and transcriptionally active state characterized by MYC activity upregulation and intron retention, which lead to the exhaustion of functional HSCs. This manifests in lymphopenia and a myeloid/megakaryocyte-skewed hematopoietic system in RXRα;RXRβ-deficient mice that eventually progresses to fatal MPD in the context of myeloproliferation-associated inflammation and hemostatic defects similar to those observed in other mouse models of fatal MPD.26,59
Chromatin openness and transcriptional activation of RXRα;RXRβ-deficient HSCs contrasts the transcriptional downregulation and reduced chromatin accessibility of RXRα;RXRβ-deficient committed cells, suggesting that the mechanisms driving RXR control over HSC quiescence and lineage choice are cell context dependent. The complexity of mechanisms that can be controlled by RXRs might be explained by their promiscuity; RXRs regulate several NRs via direct dimerization and binding of cognate ligands and coactivators, as demonstrated in other cell types (reviewed by De Bosscher et al60). Our TF motif analysis indicates that RXR/RAR binding sites are overrepresented in the LT-HSC signature, pointing to RXR/RAR signaling as an important player in RXR-mediated control of HSC fitness.5 Accordingly, recent studies demonstrated that vitamin A signaling, and especially nonclassic retinoid signaling mediated by cytochrome P450 family 26 subfamily B member (CYP26B1) and 4-oxo-RA, regulates the dormancy and LT repopulation capacity of mouse HSCs through RXR/RARβ.14 The cell-autonomous mechanism of this regulatory path may be species specific, because Cyp29b1 is expressed in mouse HSCs, whereas in humans it is expressed in BM niche but not HSC cells.13,14 Our study supports a role for RXRs in mouse HSC fitness beyond their collaborative role in RXR/RARβ heterodimers. Considering that RXRs play an active role in response to retinoids,61 remaining transcriptional effects in Rarb-knockout HSCs treated with retinoids14 might be explained by RARβ-independent RXR activation. In addition, although both Rarb- and Cyp26b1-deficient mice present diminished HSC self-renewal capacities,14 none of these mice recapitulates the profound phenotype of RXRα;RXRβ-deficient mice. For example, unlike RXRα;RXRβ-deficient LT-HSCs, HSC lacking nonclassic retinoid signaling retain radioprotection ability. This suggests that signaling pathways other than retinoids contribute to the role of RXRs in the control of LT-HSC fitness. Several recent research lines point to FAs as RXR ligands that provide a metabolic link to HSC biology. FA metabolism is specifically enriched in LT-HSCs relative to progenitor cells.14 More important, we identified the long-chain FA C24:5 as a natural ligand of RXRs in HSCs, both under basal and stressed hematopoiesis.62 Moreover, FA metabolism is upregulated in HSCs from old mice,63 and HSC functionality is recovered after treatment with y-linoleic acid in both aged humans and mice.63 Thus, although RA seems to have different effects in humans and mice,13,64 HSC responses to FA treatment are similar in both species. Future studies could explore whether combination of a retinoid with FAs could improve ex vivo expansion of human HSCs and offer a route toward stem-cell gene therapy.
Another important finding is that the loss of one Myc allele prevented the loss of quiescence and self-renewal, in vivo regeneration capacity, and the changes in the transcriptional and splicing regulatory landscapes of RXRα;RXRβ-deficient HSCs. Our results demonstrate that MYC activity lies at the root of the HSC phenotype associated with loss of RXR signaling. Interestingly, a normal distribution of progeny downstream of LT-HSCs was not restored, suggesting that MYC is indispensable for the initial phase of LT-HSC fate commitment but not for differentiation in later divisions. This is in agreement with previous studies demonstrating that MYC expression is low in dormant HSCs but increases during the transition toward active HSCs and subsequently multipotent progenitors.5 Beyond the known role of MYC in the control of self-renewal,65 our results suggest that MYC signaling activation in RXRα;RXRβ-deficient HSCs promotes a phenotype compatible with HSC aging through the control of alternative splicing, leading to global intron retention.66,67 The mechanisms by which MYC performs this transcriptional activity might be direct or indirect. MYC activation has the potential to impact HSC fitness, because alterations to ribosomal biogenesis and RNA splicing contribute to age-associated HSC epigenetic reprogramming68 and to myelodysplastic-related disorders and leukemia.69,70 Our results demonstrate that lack of RXRα;RXRβ in HSCs triggers a spike in intron retention in LT-HSCs, with extensive alteration to genes and processes involved in mRNA processing and splicing, through a MYC-dependent mechanism. Despite intense biological research over several decades, pharmacologic inhibition of MYC remains ineffective because of its “undruggable” protein structure.71 Our study points to RXRs as potential druggable targets for maintaining HSC fitness during transplantation procedures and protecting against premature HSC aging and MPD.
Acknowledgments
The authors thank the members of the J.A.C. and M.R. laboratories for extensive discussions and critiques of the manuscript. The authors thank Daniel Metzger (Université de Strasbourg, Strasbourg, France) for Rxrbf/f mice, Juan Carlos Zúñiga-Pflücker (Sunnybrook Health Sciences Centre, Toronto, ON, Canada) for OP9NL1 cells, Daniel Jiménez-Carretero (Centro Nacional de Investigaciones Cardiovasculares [CNIC]) for t-distributed stochastic neighbor embedding (t-SNE) analysis, the Centre for Genomic Regulation (CRG; Barcelona, Spain) Genomics Unit for assay for transposase-accessible chromatin with sequencing, and S. Bartlett (CNIC) for editorial assistance. The authors also thank the staff of the CNIC Cellomics and Animal facilities for technical support.
This study was supported by grants from the Spanish Ministerio de Ciencia e Innovación (MICIN) (SAF2017-90604-REDT-NurCaMein, RTI2018-095928-B100, and PID2021-122552OB-I00), La Marató de TV3 Foundation (201605-32), and the Comunidad de Madrid (MOIR-B2017/BMD-3684) (M.R.) and from the Formación de Profesorado Universitario (FPU17/01731) program (MICIN) (J.P.). The project also received funding from the US National Institutes of Health (R01 DK124115, P01 HL158688, R01 HL147536, R01 CA237016, and U54 DK126108) (J.A.C.). The Centro Nacional de Investigaciones Cardiovasculares (CNIC) is supported by the Instituto de Salud Carlos III, the Ministerio de Ciencia e Innovación, and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (grant CEX2020-001041-S funded by Spanish Ministerio de Ciencia e Innovación/Agencia Estatal de Investigación [MICIN/AEI/10.13039/501100011033]).
Authorship
Contribution: M.P.M.-G., J.P., J.S.W., J.A.C., and M.R. conceptualized the study; M.P.M.-G., J.P., R.N., A. Paredes, H.N., and V.N. performed methods; R.N., M.J.G., A. Paranjpe, A.B., D.J.S., F.S.-C., and N.S. performed software analysis; M.P.M.-G., J.P., R.N., A. Paranjpe, H.N., and M.J.G. performed data interpretation and analysis; M.P.M.-G., J.P., J.S.W., N.S., J.A.C., and M.R. wrote, reviewed, and edited the manuscript; J.A.C. and M.R. supervised the project; and J.A.C. and M.R. obtained funding.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mercedes Ricote, Centro Nacional de Investigaciones Cardiovasculares (CNIC), Melchor Fernández Almagro, 3, 28029 Madrid, Spain; e-mail: mricote@cnic.es; and Jose A. Cancelas, Hoxworth Blood Center, 3130 Highland Ave, Cincinnati, OH 45267; e-mail: jose.cancelas@cchmc.org.
References
Author notes
Transcriptomic data are deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus, with accession numbers GSE191163 and GSE199937.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
M.P.M.-G. and J.P. contributed equally to this study.

![Myc haploinsufficiency rescues regeneration, transcriptome, and spliceosome of phenotypically identifiable LT-HSCs. (A-B) Competitive transplantation assay of unfractionated BM cells from 4-month-old WT, VavCre+Rxrabfl/fl, and VavCre+Rxrabfl/fl-Myc+/fl mice (CD45.2+) in a 1:1 ratio with C57BL/6 BM cells (CD45.1+) into lethally irradiated C57BL/6 mice (CD45.1+) (data pooled from 2 independent experiments). (A) Experimental scheme. (B) Percentages of CD45.2+ BM LT-HSCs at 16 weeks after transplantation. (C) Frequencies of CD150+CD48− LT-HSCs from WT, VavCre+Rxrabfl/fl, and VavCre+Rxrabfl/fl-Myc+/fl mice in the G0 phase of the cell cycle (pyronin Y−/Hoechst 33342−; data pooled from 3 independent experiments). (D) Percentage of 5-bromo-2′-deoxyuridine–positive (BrdU+) CD150+CD48− LT-HSCs from WT, VavCre+Rxrabfl/fl, and VavCre+Rxrabfl/fl-Myc+/fl mice after 1-hour pulse with BrdU. (E-F) Immunofluorescence analysis, showing different division modes according to MYC expression in paired daughter LT-HSCs from WT, VavCre+Rxrabfl/fl, and VavCre+Rxrabfl/fl-Myc+/fl mice. (E) Representative images showing (1) symmetric self-renewal (absence of MYC expression in paired daughter cells), (2) asymmetric division (high vs low MYC expression in paired daughter cells), and (3) symmetric differentiation/commitment (MYC expression in both cells); scale bar, 10 μm. (F) Percentage of LT-HSCs corresponding to division modes 1, 2, and 3 as in E; n = 60 division pairs captured from 5 WT mice; n = 48 division pairs captured from 3 VavCre+Rxrabfl/fl mice; and n = 37 division pairs captured from 5 VavCre+Rxrabfl/fl-Myc+/fl mice. Analyses were performed in 2 independent experiments. (G) Serial CFU plating assay in total BM cells from WT, VavCre+Rxrabfl/fl, or VavCre+Rxrabfl/fl-Myc+/fl mice. (H-L) Bulk transcriptome of WT, VavCre+Rxrabfl/fl, and VavCre+Rxrabfl/fl-Myc+/fl LT-HSCs. Normalized expression values from bulk RNA-seq data are provided in supplemental Tables 12-15. (H) Rescued differentially expressed genes in VavCre+Rxrabfl/fl-Myc+/fl vs VavCre+Rxrabfl/fl LT-HSCs (eBayes t-test P < .05; fold > 1.2), compared with VavCre+Rxrabfl/fl-Myc+/fl vs WT LT-HSCs (eBayes t-test P < .05; false discovery rate corrected; fold > 1.5). (I) Heat map of the 435 rescued genes showing silencing of VavCre+Rxrabfl/fl LT-HSC–induced transcripts by Myc+/fl expression. On the left are shown example rescued genes (Figure 5D; TFs or RNA-binding regulators). (J) Rescued alternative splicing events in VavCre+Rxrabfl/fl-Myc+/fl vs VavCre+Rxrabfl/fl LT-HSCs, compared with VavCre+Rxrabfl/fl-Myc+/fl vs WT LT-HSCs (eBayes t-test P < .05; δ percentage spliced in [PSI] > 0.1 for both comparisons). (K) Heat map of 154 unique splicing events, organized by increased junction PSI value in VavCre+Rxrabfl/fl vs WT LT-HSCs, with exemplar intron retention events (green tick mark) and genes symbols called out. (L) Number of observed unique annotated splicing events in the 154 rescued splicing events, by exon/intron inclusion or exclusion. Splicing events associated with exon or intron inclusion (increased relative expression) vs exclusion are shown separately. Results are presented as means ± SEM, and dots represent individual animals. Significance was determined by ordinary 1-way analysis of variance, and is represented as follows: ∗P ≤ .05, ∗∗P ≤ .01, and ∗∗∗P ≤ .001. DAPI, 4′,6-diamidino-2-phenylindole; n.s., not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/141/6/10.1182_blood.2022016832/6/m_blood_bld-2022-016832-gr7.jpeg?Expires=1770283215&Signature=sjq8S0VJFCwNhiYqPiOcQLxsHyBJJBsU0QT7qzj~EtE4f-MOb-pLtMFPyMfb5rj-StkdblbqB5dWwozkpkduhnxVK4z8PS7nkI3NmQoAayPWSLsNpf0ou9Gvg9HxZVIMJDtpb7iCe~9fSfYXPedV9qzGCrsCjgbhl~YtmCgl~DH1yFJswYAk~o7bIn-ar8qlvgWF5Q8jiSHmILz0aSUlBABzNvHw6aR94Eo4cvfEj-AVP-PCFlxAGvXXraRpNp8mO5lVliOKt-OFW6BFUVKb4sBmt0mHIIs-pVvMZncJ0bYdwMDE4hQSutyavxHmMPiRolh8iKZoceIaBrC7UsAXmg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal