TO THE EDITOR:
Allogeneic hematopoietic stem cell transplantation (HSCT) is a curative therapy for the majority of hematological malignancies but is limited by graft-versus-host disease (GVHD) and opportunistic infections. Severe acute GVHD in the gastrointestinal (GI) tract is a major determinant of mortality1 and causes progressive epithelial and crypt loss, leading to denudation of intestinal mucosa.1-3 Approaches to prevent severe GVHD to date focus on the immune suppression of T cells with calcineurin inhibitors, antimetabolites, or alkylating agents.3
Intestinal stem cell (ISC) function and associated intestinal epithelial cell (IEC) renewal are regulated by signaling pathways of WNT, R-spondin, Notch, bone morphogenetic proteins, and epidermal growth factor.4 Stem cell populations expressing leucine-rich repeat-containing G protein−coupled receptor 5 (LGR5) are essential in the renewal of intestinal epithelium during homeostasis. R-spondins are encoded by 4 genes (rspo1, 2, 3, and 4) and represent the cognate ligands for LGRs, including LGR5, which enhance WNT-β-Catenin-TCF4 signaling. R-spondin 1 and 3 have known protective effects in preventing experimental GVHD by promoting the repopulation of ISCs and Paneth cells, confirming that strategies to promote ISC renewal and epithelial regeneration represent promising approaches to prevent and treat severe gut GVHD.5-8 Overexpression of R-spondins, however, can also promote tumorigenesis, and mutations in R-spondin or associated regulatory genes have been identified in 10% of colorectal cancers, raising potential limitations for the therapeutic use of R-spondin.9
Although lithium has been used as a highly effective treatment for bipolar disorders since the 19th century, our understanding of the mechanism of action in this setting remains limited.10 Broadly, lithium directly inhibits the following: (1) inositol monophosphatase and phosphomonoesterases that share a metal ion binding consensus sequence, (2) phosphoglucomutase that catalyzes the conversion between glucose-1-phosphate and glucose-6-phosphate, and (3) glycogen synthase kinase-3 (GSK-3), a pluripotent kinase that antagonizes WNT signaling. Lithium thus stimulates WNT/β-catenin signaling via GSK-3 inhibition.10,11 Findings that the WNT/β-catenin pathway drives cell proliferation and epithelial regeneration and that long-term use of lithium is not associated with an increased risk of colorectal cancer suggest that inhibition of GSK-3 may be clinically tractable.12
In a clinical pilot study conducted at the Fred Hutchinson Cancer Research Center and University of Washington, 20 patients with steroid-refractory gut GVHD and mucosal denudation at endoscopy were treated with oral lithium carbonate in addition to second-line therapy. Ten of the 20 patients (50%) had a complete remission, and 7 (35%) survived more than 2 years.13 These results were encouraging, because a historical review showed that only 10% of the patients with steroid-refractory stage 3 to 4 intestinal GVHD at the Fred Hutchinson Cancer Research Center survived for more than 2 years.13
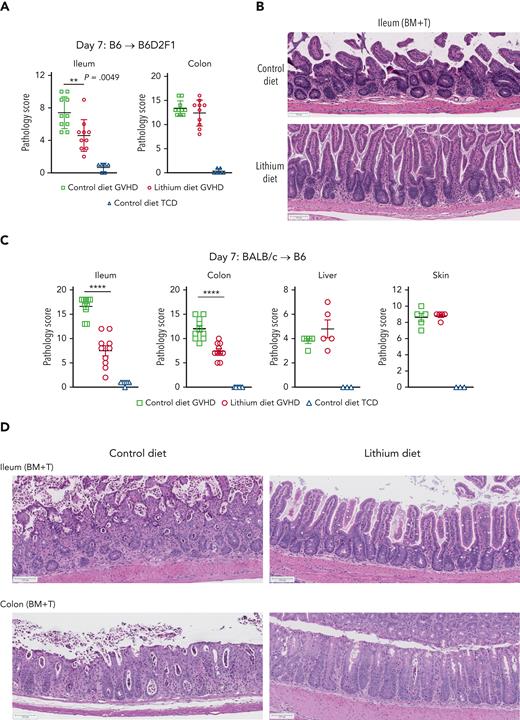
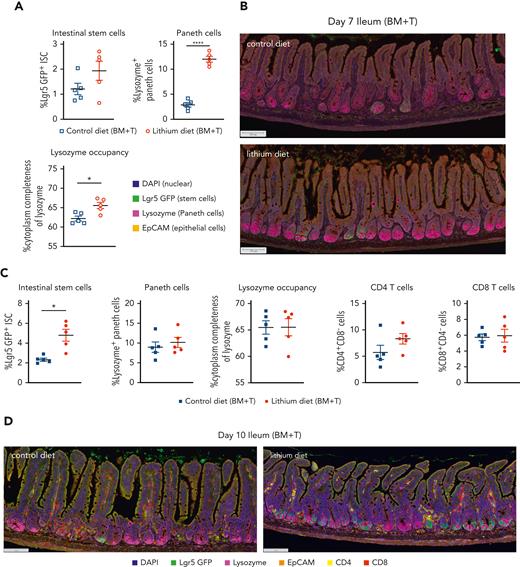
To test the premise that the clinical effect of lithium was related to enhanced mucosal regeneration, we evaluated the effects of lithium on GI tract histopathology in a murine model of GVHD. The presence of donor T cells in the graft caused severe tissue injury in the ileum and colon of irradiated allogeneic recipients in 2 MHC disparate models of acute GVHD (Figure 1A-D). Administration of lithium chloride (LiCl) from 2 days before HCT to day 7 after T cell−replete HCT consistently decreased GVHD in the ileum in both models. Attenuation of GVHD in the colon was also seen in the BALB/c → B6 system (Figure 1C-D). Lithium administration did not have an impact on GVHD in the liver or skin (Figure 1C-D). Crypt apoptosis and loss in the ileum was consistently attenuated by LiCl treatment (supplemental Figure 1A-B, available on the Blood website). The lack of consistent effects of lithium on GVHD in organs other than the ileum suggested a tissue-specific effect rather than broad effects on alloreactive donor T-cell function. Although LiCl had no effect on the percentage of Lgr5+ ISCs in the ileum on day 7, the percentage of Paneth cells in the ileum and the amount of intracellular lysozyme, an antimicrobial enzyme, per Paneth cell were significantly increased by lithium treatment (Figure 2A-B). These findings are consistent with previous studies showing that Wnt signaling is critically required for Paneth cell maturation14 and that the administration of the Wnt agonist R-spondin1 promotes intestinal stem cell differentiation to Paneth cells after HSCT.5 Of note, Paneth cells constitutively reside at the base of crypts of the small intestine, whereas they are found in the other parts of GI tract such as the stomach and colon only during inflammation.15 Thus, the protective effect of LiCl administration found primarily in the ileum is congruent with the systemic distribution of Paneth cells. As might be predicted if the effects of lithium were localized to the intestinal stem cell niche, the effects were also seen in non-GVHD recipients of T-cell−depleted grafts (supplemental Figure 2A-B). Interleukin-22 (IL-22), produced by type 3 innate lymphoid cells,16 and Reg3γ, which is produced by crypt cells during GVHD,17 were not systemically altered by LiCl administration (supplemental Figure 1C-D), consistent with effects of lithium that are likely independent of these molecules.
Lithium attenuates gut GVHD. (A-B) Lethally irradiated (1300 cGy) B6D2F1 recipients were transplanted with bone marrow (BM) (5 × 106) with or without purified splenic T cells (3 × 106). Recipients received a normal control or lithium-containing diet (0.2% from day −2 to +2 then 0.4% from day 3 onward). Semi-quantitative GVHD histopathology in (A) ileum (left) and colon (right) at day +7 after HSCT. (B) Representative hematoxylin and eosin images of ileum. (C-D) Wild-type B6 recipients were lethally irradiated (1000 cGy) and transplanted with BALB/c BM (10 × 106) and purified splenic T cells (5 × 106) with control or lithium diet treatment as described above. (C) Semiquantitative GVHD histopathology in ileum, colon, liver, and skin at day +7 after HSCT. (D) Representative hematoxylin and eosin images of ileum and colon. Scale bar, 100 μm. n = 3 to 10 per group. GI tract from 2 replicate experiments; liver and skin from 1 experiment. Data are presented as mean ± standard error of the mean (SEM). ∗∗P < .01; ∗∗∗∗P < .0001.
Lithium attenuates gut GVHD. (A-B) Lethally irradiated (1300 cGy) B6D2F1 recipients were transplanted with bone marrow (BM) (5 × 106) with or without purified splenic T cells (3 × 106). Recipients received a normal control or lithium-containing diet (0.2% from day −2 to +2 then 0.4% from day 3 onward). Semi-quantitative GVHD histopathology in (A) ileum (left) and colon (right) at day +7 after HSCT. (B) Representative hematoxylin and eosin images of ileum. (C-D) Wild-type B6 recipients were lethally irradiated (1000 cGy) and transplanted with BALB/c BM (10 × 106) and purified splenic T cells (5 × 106) with control or lithium diet treatment as described above. (C) Semiquantitative GVHD histopathology in ileum, colon, liver, and skin at day +7 after HSCT. (D) Representative hematoxylin and eosin images of ileum and colon. Scale bar, 100 μm. n = 3 to 10 per group. GI tract from 2 replicate experiments; liver and skin from 1 experiment. Data are presented as mean ± standard error of the mean (SEM). ∗∗P < .01; ∗∗∗∗P < .0001.
Lithium mitigates early paneth cell loss in the ileum by enhancing lgr5+intestinal stem cell recovery. B6 Lgr5-GFP transgenic recipients were lethally irradiated (1000 cGy) and transplanted with BALB/c BM (10 × 106) and purified splenic T cells (5 × 106) with control or lithium diet treatment as described above. Ileum analysis at day +7 (A-B) and day +10 after HSCT (C-D) are shown. (A,C) Proportions of Lgr5-GFPhi EpCAM+ ISC and lysozyme+ EpCAM+ Paneth cells in the ileum crypt, and the average of cytoplasmic lysozyme size in Paneth cells are shown. (C) Proportions of CD4+CD8neg cells and CD8+CD4neg cells in the ileum crypt are shown. (B,D) Representative multispectral images. Scale bar, 100 μm. Data at day 7 or day 10 (n = 5 per group). Data are presented as mean ± SEM. ∗P < .05; ∗∗∗∗P < .0001.
Lithium mitigates early paneth cell loss in the ileum by enhancing lgr5+intestinal stem cell recovery. B6 Lgr5-GFP transgenic recipients were lethally irradiated (1000 cGy) and transplanted with BALB/c BM (10 × 106) and purified splenic T cells (5 × 106) with control or lithium diet treatment as described above. Ileum analysis at day +7 (A-B) and day +10 after HSCT (C-D) are shown. (A,C) Proportions of Lgr5-GFPhi EpCAM+ ISC and lysozyme+ EpCAM+ Paneth cells in the ileum crypt, and the average of cytoplasmic lysozyme size in Paneth cells are shown. (C) Proportions of CD4+CD8neg cells and CD8+CD4neg cells in the ileum crypt are shown. (B,D) Representative multispectral images. Scale bar, 100 μm. Data at day 7 or day 10 (n = 5 per group). Data are presented as mean ± SEM. ∗P < .05; ∗∗∗∗P < .0001.
Protection and maintenance of intestinal stem cells are crucial to repair severe ulceration and sloughing of the epithelium observed in patients with denuded intestinal mucosa13 and to regenerate intestinal epithelia. Previous studies have shown that GVHD causes Paneth cell injury that, in turn, enhances luminal dysbiosis and ISC damage.5,18 Low Paneth cell numbers at the onset of GVHD are also associated with high transplant-related mortality.19 Because Paneth cell function within the ISC niche is essential for the maintenance of ISCs,20 we evaluated the abundance of ISC at a later time point. We chose day 10 after transplantation because recipients of T-cell−replete grafts typically succumb to GVHD shortly thereafter. Compared to the vehicle control, administration of LiCl significantly increased the percentage of Lgr5+ ISCs in the ileum (Figure 2C-D). Importantly, at this time point, earlier effects of LiCl on Paneth cells had abated due to recovery in the control recipient’s ileum (Figure 2C-D), suggesting that the primary effects of LiCl on Paneth cells subsequently promoted ISC recovery. In addition, LiCl had no effect on the percentages of CD4+ T cells or CD8+ cells in the ileum (Figure 2C-D), although effects on the function of donor T cells cannot be excluded. In sum, the observed effects of lithium on Paneth cells and ISCs are consistent with effects that are focused on the intestinal stem cell niche.
These findings support the premise that agents targeted to the intestinal stem cells niche are likely to represent an effective adjunctive prevention and treatment of severe gut GVHD. Importantly, GSK3 inhibition with lithium offers a simple approach to treatment of this barrier dysfunction disorder and is consistent with the effects seen with IL-22 and IL-29 that also target the stem cell niche.16,21 Given the increase of Paneth cells and their antimicrobial lysozyme contents, lithium administration may alter the microbiota and metabolites in the GI tract, which may also modify systemic GVHD. In all, 45% of patients experienced mental status changes as adverse effects of lithium in our previous pilot study.13 Furthermore, drug absorption may fluctuate depending on GI tract integrity, and so the monitoring of serum concentrations and psychiatric adverse effects is required during lithium treatment. The discovery of selective GSK3 inhibitors may circumvent these psychiatric adverse effects and permit long-term therapy.
Our findings encourage further studies of lithium, other GSK3 inhibitors, and Wnt activators in GVHD. Furthermore, agents that target this axis may also be useful as GVHD prophylaxis approaches.
Acknowledgments
The authors thank S.J. Weaver and A.L. Koehne in Experimental Histopathology for expert preparation of histology samples.
G.R.H. and M.K. are supported by R01 HL148164. Experimental histopathology at Fred Hutchinson Cancer Research Center was supported by the National Institutes of Health (NIH)/National Cancer Institute (P30CA015704).
The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Authorship
Contribution: G.R.H. and M.K. conceived and designed the studies; M.K., L.S., K.S.E., and S.T. conducted experiments; M.K. analyzed results and interpreted data; P.J.M. reviewed and revised the manuscript; A.D.C. undertook blinded histopathology assessment of murine tissues; G.R.H. and M.K. wrote the manuscript; and all authors reviewed, critically edited, and approved the final manuscript for submission.
Conflict-of-interest disclosure: P.J.M. has research support from AltruBio. G.R.H. has consulted for Generon Corporation, NapaJen Pharma, iTeos Therapeutics, and Neoleukin Therapeutics, and has received research funding from Compass Therapeutics, Syndax Pharmaceuticals, Applied Molecular Transport, Serplus Technology, Heat Biologics, Laevoroc Oncology, and iTeos Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Motoko Koyama, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: mkoyama@fredhutch.org; and Geoffrey R. Hill, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: grhill@fredhutch.org.
References
Author notes
Data available from corresponding author on request.
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal