Abstract
Diffuse large B-cell lymphoma (DLBCL) is an aggressive but potentially curable disease and is most common in older people. Rituximab-CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) is the standard of care for fit patients without cardiac contraindications. In each individual older patient, the potential gains of treatment should be balanced against the risks of treatment-related morbidity and mortality. A simplified comprehensive geriatric assessment or easily performed assessments such as gait speed and grip strength can be helpful to assess the fitness of an older patient. Prephase with corticosteroids, rigorous supportive care including granulocyte colony-stimulating factor prophylaxis and careful monitoring can be important in preventing adverse events. In unfit older patients, a dynamic dosing strategy is often applied. For very old patients (≥80 years) a dose-reduced regimen (rituximab-miniCHOP) is recommended. When anthracyclines are contraindicated, doxorubicin can be replaced by etoposide or gemcitabine. Most frail patients do not benefit from chemotherapy. Further progress can be expected from non-chemotherapy-based therapies, such as bispecific antibodies, antibody-drug conjugates, and immunomodulatory agents. This article provides an overview of first line treatment in older patients with DLBCL and our approach to the management of these challenging cases.
Case presentation 1
A 77-year-old man with mild hypertension, paroxysmal atrial fibrillation, and a low-risk prostate carcinoma for which a wait-and-see policy was followed, presented with an enlarged lymph node in the left neck and swelling of the lingual tonsils. No clinical symptoms were reported. The ear, nose, and throat specialist suspected a tonsil carcinoma and performed a biopsy of the lingual tonsil and an incision biopsy of the enlarged lymph node. The intubation procedure was complicated by bleeding and swelling of the lingual tonsils, and resulted in a threatened airway and admission to the intensive care unit. He was treated with dexamethasone and could be extubated 3 days later. Diffuse large B-cell lymphoma (DLBCL), germinal center B-cell (GCB) subtype was diagnosed. Staging procedures, including fluorodeoxyglucose (FDG)-positron emission tomography (PET) and computed tomography (CT) scans of the neck, chest, and abdomen with contrast, revealed Ann Arbor stage III disease with localizations in lymph nodes above and below the diaphragm and lingual tonsils. The patient had an International Prognostic Index (IPI) score of 3 and a central nervous system (CNS) IPI score of 3. Cardiac pretreatment evaluation at the oncocardiology unit showed a left ventricular ejection fraction (LVEF) of 60%. He received a prephase treatment consisting of prednisone (100 mg daily for 5 days) followed by 6 cycles of R-CHOP (rituximab combined with cyclophosphamide, doxorubicin, vincristine, and prednisone; all full doses) plus 2 additional cycles of rituximab and granulocyte colony-stimulating factor (G-CSF) support. The first cycle was complicated by neutropenic fever and atrial fibrillation, for which he was admitted to the hospital and treated with broad spectrum antibiotics and an antiarrhythmic agent. The remaining R-CHOP cycles were uneventful. The PET scan after the sixth R-CHOP cycle revealed a complete remission. Two years after treatment, the patient is still in remission.
Case presentation 2
An 84-year-old man was referred to our hospital for treatment of a newly diagnosed Ann Arbor stage III, activated B-cell (ABC) subtype DLBCL with supraclavicular, bilateral axillar, and para-iliac lymph nodes. He had lost 5 kg in 3 months and since the start of his symptoms his World Health Organization (WHO) performance status had decreased to 2, limiting him in his usual daily activities. Before the start of his illness, he had been fit, activities of daily living (ADL)-independent and cycled 10 km 3 to 4 times a week. His prior medical history revealed type 2 diabetes mellitus complicated by chronic renal failure (estimated glomerular filtration rate of 40 mL/min). Geriatric assessment was performed using the cumulative illness rating scale-geriatric (CIRS-G) assessment tool in which he scored 3 based on minor renal impairment and diabetes, controlled by oral antidiabetics. Cardiac evaluation showed an LVEF of 52%. Because his premorbid condition had been exceptionally good, his CIRS-G score was low, and he had a good LVEF, we decided to treat him with curative intent. He was treated with a prephase of prednisone, followed by 6 cycles of R-miniCHOP and 2 additional cycles of rituximab. The patient received pegfilgrastim (6 mg) subcutaneously on day 2 of each cycle. The first 3 courses were uneventful, and, despite this, we decided not to escalate the dose to full-dose R-CHOP, given his high age. Interim response evaluation by CT scan showed a good partial remission. The sixth cycle was delayed for 1 week due to persistent thrombocytopenia that resolved spontaneously. No further dose reduction was applied. The PET scan performed 6 weeks after the final course showed a complete metabolic response. There were no signs of recurrence in the 18 months after the last rituximab cycle.
Introduction
DLBCL is the most common type of non-Hodgkin lymphoma (NHL), representing 25% to 30% of all cases in the developed world.1 The incidence of DLBCL in the United States is ∼7 cases per 100 000 persons per year.2 It can develop at any age but is more common in older individuals. Notably, DLBCL is most frequent in individuals aged 65 to 74 years, and ∼29% of cases are in individuals ≥75 years.2 The median age at diagnosis is 66 years. As the population ages, DLBCL is expected to become more prevalent.
DLBCL is an aggressive but potentially curable disease. The overall survival (OS) of patients with DLBCL has improved significantly after the addition of rituximab to standard combination chemotherapy such as CHOP.3,4 Real-world data from The Netherlands show that trends in 5-year survival have improved over the last 2 decades. The prognosis for DLBCL has improved for all age groups but less so for those >75 years of age. A considerable proportion of older patients do not receive any treatment (17%, in patients aged 65-74 years; and 36%, in patients aged ≥75 years vs 10%, in patients aged <65 years). However, when untreated patients were excluded, patients aged >75 years had a similar increase in survival to younger patients.5 Nevertheless, older patients still have a worse outcome compared with younger patients and this, most likely, is of multifactorial origin. Older patients may well be undertreated because prescribers are fearful of chemotherapy-induced toxicities, because loss of bone-marrow function and altered drug pharmacokinetics and pharmacodynamics can increase the number of treatment-related adverse events.6 Another Dutch population–based study demonstrated that two-thirds of patients with NHL aged >60 years have ≥1 comorbidity. Cardiovascular disease occurs most often and has a strong negative prognostic influence. Patients with ≥2 comorbidities had a significantly lower OS than patients with no comorbidities (hazard ratio, 1.9). Moreover, patients with more comorbidities were less likely to be treated with curative intent.7 Anthracycline-based chemotherapy improves survival in DLBCL but can induce cardiotoxic effects and is therefore often not given to older patients. Older patients are often excluded from trial participation based on their chronological age alone and only 5% of trials in hematological malignancies focus exclusively on older patients.8
There are challenging dilemmas in managing older patients with DLBCL that raise the following questions: (1) how to define older patients, (2) is the disease in older patients biologically different from DLBCL in younger patients, (3) how to utilize practical tools to accurately assess the fitness of a patient in daily practice, (4) how to support older patients with DLBCL during immunechemotherapy, and (5) how to treat a patient with contraindications for anthracyclines? In this review we present 2 cases that highlight these dilemmas, and we provide guidance on clinical management of first-line therapy in older patients with DLBCL.
How do we define older patients?
In developed countries, for statistical and public administrative purposes, “old age” is frequently defined as ≥65 years of age. There is no clear medical or biological evidence to support this definition. Chronological age is an easy-to-determine number; however, wide differences exist in health at a given chronological age. Some adults become frail in early old age, whereas others remain fit into their 90s and beyond.9 An American study among people aged 51 to 85+ years demonstrated that a significant proportion of the older population is living healthy and active lives at all ages, including among those aged ≥85 years. However, that there is also a broad variation in quality of life within every age group, with individuals in good and poor health present at both ends of the age spectrum.10 The International Society of Geriatric Oncology and the European Society for Medical Oncology (ESMO) suggest that the term “older” or “old” should be used for all patients aged >70 years, yet, chronological age alone is a poor predictor of cancer treatment tolerance.11 The heterogeneity of older patients with lymphoma necessitates a carefully tailored approach to care that considers individual frailty. Risk assessment based on potential outcome and comorbidities is an essential part of medicine for older ages.
Is the disease different from DLBCL in young patients?
Besides the prevalence of comorbidities associated with advanced age and its effect on treatment regimens, potential contributing factors in the adverse prognosis of DLBCL in older patients are the intrinsic biological features of the tumor, as well as the immune response against these tumors. Although there seems to be no major difference in histopathological features, there are some differences that deserve attention.
The stromal gene signature in large B-cell lymphoma (GCB/ABC) has long been established as an independent determinant of prognosis.12
Several reports have demonstrated that the relative incidence of ABC vs GCB subtype significantly increases with age. In a small, age-matched case-control analysis of ABC vs GCB subtype, the ABC subtype remained correlated with poor survival.13,14 Interestingly, the direct opposite has been observed in pediatric DLBCL, which is associated with GCB subtype.15 The difference in distribution of ABC subtype with advanced age remains speculative but several mechanisms have been proposed, all associated with immune senescence observed in the older. Decrease in B-cell receptor diversity has been observed in advanced age and this is characterized by clonal expansion of B cells, in vivo.16 An example of this expansion is the increase in the number of B cells expressing the VH4-34 IgH seen with aging.17 Strikingly, DLBCL expressing this gene is typically classified in the ABC subtype.18 These findings suggest that the malignant DLBCL clone evolves in the background of clonal expansion of B cells associated with aging.
Another condition that is associated with advanced age is the Epstein-Barr virus (EBV)-positive DLBCL, not otherwise specified, previously termed EBV-positive DLBCL of the older. Although this disease can be seen in younger patients, it is usually seen in older patients and is associated with immune senescence. It develops in the absence of primary or secondary immune deficiency and usually presents with EBV latency pattern III, as is usually seen in immune deficient states such as post-transplant lymphoma and advanced HIV infection.19 EBV-positive DLBCL is mostly of the ABC subtype, patients tend to have high IPI scores and more extranodal involvement, and generally have unfavorable outcome compared with EBV-negative DLBCL.
Unfortunately, neither one of these differences is yet reflected in different management and whereas prognosis is different, treatment remains the same for these different subgroups.
Diagnostic workup and response assessment
For older patients treated with curative intent, the diagnostic work-up should be as complete as for any young patient. The diagnosis should ideally be based on an excisional biopsy of an abnormally enlarged lymph node or extranodal tissue biopsy. Processing by an expert hematopathology laboratory with full diagnostic capabilities (immunohistochemistry and molecular) is essential. EBV confirmation by EBER-1 staining is helpful to confirm or exclude a diagnosis of EBV-positive DLBCL. At our institution, fluorescent in situ hybridization (FISH) for genetic rearrangements in MYC is performed in every patient newly diagnosed with DLBCL. In case of a positive MYC FISH, additional FISH is done for BCL2 and BCL6 rearrangements. Patients with genetic rearrangements in c-MYC in addition to BCL2 and/or BCL6 have high-grade B-cell lymphoma with translocations involving MYC and BCL2 and/or BCL6 according to the WHO 2016 classification (double-hit/triple-hit disease). Patients with MYC double-hit/triple-hit disease in which MYC is translocated to an IG partner have a dismal prognosis.20,21 The distinction between GCB subtype and ABC subtype, studied by gene expression profiling and suggested by immunohistochemistry, do not influence treatment choices at the moment. Laboratory testing includes complete blood cell counts and serum testing for renal and liver function, lactate dehydrogenase and serology for HIV and hepatitis B and C. Staging should be with PET-CT scans. We only perform bone marrow aspiration and biopsy in case of a negative PET scan if the result would influence the management of the lymphoma. All patients who are candidates for anthracycline-based treatment have baseline LVEF estimation, preferably by echocardiography. Assessment of end-of-treatment remission should be based on PET-CT according to the Lugano criteria, using the 5-point scale.22
How to assess the fitness of the patient
Although several geriatric assessment tools are available to assess fitness of patients, these are not widely applied in routine practice. Comprehensive geriatric assessments such as CIRS-G are time consuming, often require a geriatrician, and are not validated to guide treatment. Furthermore, most of these historically used assessments are static and do not measure whether treatment results in return of vitality.
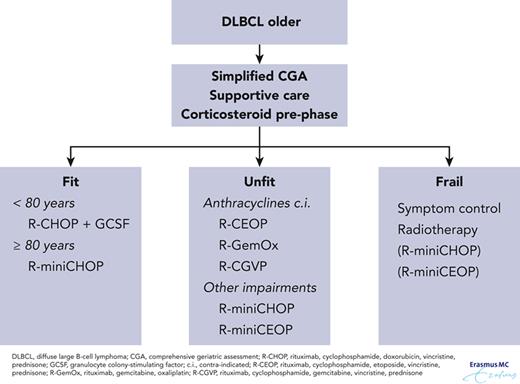
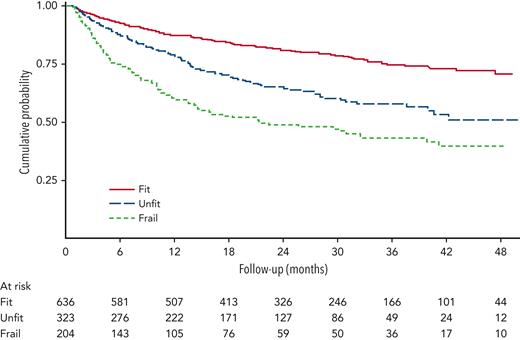
The Italian Lymphoma Foundation (FIL) has defined and validated the older prognostic index (EPI) based on a simplified comprehensive geriatric assessment (sCGA) that incorporates ADL, instrumental ADL, CIRS-G, and age for patients treated for aggressive lymphoma. In a prospective registry study of 1353 patients, the sCGA was applied to divide older patients aged >65 years into 3 groups, fit, unfit, and frail (Table 1). Treatment choice was left to the physicians’ discretion. When sCGA was integrated with age <80 years or ≥80 years, OS in the different groups was significantly different. The 3 EPI groups had different 3-year OS, 87% (95% confidence interval [CI], 81-91), 69% (95% CI, 63-73), and 42% (95% CI, 36-49) in the low-, intermediate- and high-risk groups, respectively (Figure 1). Curative intent treatment was classified according to anthracycline doses, with doses >70% classified as curative approach, doses <70% as intermediate, and palliative if anthracyclines had been omitted. This study demonstrated that fit patients benefit from curative intent treatment and that in unfit patients an intermediate dose treatment was not significantly associated with worse outcome compared with curative intent but was superior to omission of anthracyclines.23
Criteria for simplified geriatric assessment
| Criteria . | Fit . | Unfit . | Frail . | |
|---|---|---|---|---|
| ADL | ≥5∗ | <5∗ | 6∗ | <6∗ |
| IADL | ≥6∗ | <6∗ | 8∗ | <8∗ |
| CIRS-G | 0 score = 3-4 or ≤8 score = 2 | ≥1 score = 3-4 or >8 score = 2 | 0 score = 3-4 or <5 score = 2 | ≥1 score = 3-4 or ≥5 score = 2 |
| Age | <80 | <80 | ≥80 | ≥80 |
| Criteria . | Fit . | Unfit . | Frail . | |
|---|---|---|---|---|
| ADL | ≥5∗ | <5∗ | 6∗ | <6∗ |
| IADL | ≥6∗ | <6∗ | 8∗ | <8∗ |
| CIRS-G | 0 score = 3-4 or ≤8 score = 2 | ≥1 score = 3-4 or >8 score = 2 | 0 score = 3-4 or <5 score = 2 | ≥1 score = 3-4 or ≥5 score = 2 |
| Age | <80 | <80 | ≥80 | ≥80 |
ADLs include independence in (1) bathing, (2) dressing, (3) toileting, (4) getting in/out of bed, (5) continence, and (6) feeding. IADLs include independence in (1) using telephone, (2) shopping, (3) preparing own meals, (4) housework, (5) laundry, (6) using transportation, (7) taking own medication, and (8) managing money. CIRS-G categories include (a) cardiac, (b) vascular, (c) hematologic, (d) respiratory, (e) ear, nose, and throat, (f) upper gastrointestinal, (g) lower gastrointestinal, (h) hepatobiliary, (i) renal, (j) genitourinary, (k) musculoskeletal and skin, (l) neurologic, (m) endocrine/breast, and (n) psychiatric. A score of 2 indicates need for daily treatment or moderate disability/morbidity; a score of 3 indicates poor control with first-line therapy, significant disability, severe problem, or organ failure.
IADL, instrumental activities of daily living; CIRS-G, cumulative illness rating scale for geriatrics
Number of residual functions.
Overall survival by simplified geriatric assessment in all patients with treatment details (N = 1163). Adapted from Merli et al23 with permission.
Overall survival by simplified geriatric assessment in all patients with treatment details (N = 1163). Adapted from Merli et al23 with permission.
In an earlier study by FIL,24 patients were treated with dose reduction according to sCGA, which resulted in satisfactory outcome and low toxicity. It should be noted that in the previous study, all patients were classified as frail if aged >80 years whereas, obviously, there are patients aged >80 years who could benefit from more than palliative care. Although randomized controlled trials are needed to confirm these data, they do offer a reasonable approach to quantify a geriatric assessment for treatment of lymphoma.
We have not routinely performed these practices ourselves in the past but have started doing it because there are robust data in support of these practices. When in doubt, we do refer to a geriatrician.
Recent studies have reported on other, more easily incorporated assessments, such as gait speed and grip strength, as useful parameters that could be utilized to guide treatment intensity.25
Gait speed is an easily performed assessment in which an individual is required to walk 4 m and the speed is recorded with a stopwatch. Grip strength is a test in which the strength in the dominant hand is measured in kilograms using a dynamometer and it can be performed for nonambulatory patients. A study performed by Liu et al showed that gait speed identifies frailty and predicts outcomes independently of performance status among older patients with blood cancers and that every 5-kg decrease in grip strength was associated with worse survival (adjusted hazard ratio [HR], 1.24; 95% CI, 1.07-1.43) but not with hospital or emergency department use.25
Both tests have not been validated prospectively but their prognostic value combined with the ease in which they are performed render them attractive ways to assess frailty.
An important but often overlooked factor in daily practice is nutritional status of patients; yet malnutrition occurs in at least 10% of older patients. Lee et al reported on a geriatric nutritional risk index in older patients with DLBCL, consisting of body mass index and serum albumin that identifies patients at higher risk of mortality owing to poor nutritional status.26 This simple and easily applied tool can be used to identify patients in whom consultation of a dietician could be beneficial.
In a recently published study, patients aged >75 years who were transplant ineligible were randomized to receive standard oncological care or care with additional geriatric consultation. The intervention did not improve survival but did improve the odds of having end-of-life goals-of-care discussion.27
Therefore, although there are numerous ways of assessing the fitness of an older patient, as outlined earlier, for now they only serve the purpose of objectively categorizing patients. Recommending a specific strategy is therefore difficult. Geriatric assessment scales have been studied most but simply applied tests, such as grip strength, could more easily be incorporated into routine care.
How do we treat a fit, older patient with DLBCL?
R-CHOP every 3 weeks (R-CHOP-21) is considered standard of care for older patients with DLBCL. A large randomized trial demonstrated that 8 cycles of R-CHOP-21 and 8 cycles of R-CHOP-14 have similar efficacy and toxicity in patients aged 60 to 80 years (Table 2).28 Although there are no data from prospective randomized trials to illustrate that 6 cycles of R-CHOP-21 are as effective as 8 cycles, results from 2 population-based studies and exploratory analysis of the GOYA trial support the hypothesis that 6 cycles of R-CHOP-21 might be as effective as 8 cycles in both young and older patients and result in the same OS.35-37 Six cycles of R-CHOP-21 has a reduced exposure and shorter duration of treatment compared with 8 cycles. In a study in Germany, older males had unfavorable rituximab pharmacokinetics and worse outcome than older female patients with DLBCL.38,39 In the SEXIE-R-CHOP-14 study, older males received a one-third higher dose of rituximab than females. The study showed that, when compared with a historical control group, the adverse prognosis of older male patients was abrogated.40 The OPTIMAL>60 study, in which higher rituximab serum levels and exposure times for males and females are being investigated in a randomized approach in older patients with DLBCL, will provide more data to support a higher rituximab dose in male patients. Recently, the POLARIX study demonstrated that treatment with polatuzumab vedotin-R-CHP had a significant but small progression-free survival (PFS) benefit over R-CHOP. However, the complete remission rate did not improve and there was no overall survival benefit. The study was not powered to compare PFS in subgroups but the data did suggest that patients aged >60 years had a benefit.41 Because of the lack of OS benefit, we believe that R-CHP will not become standard of care. We therefore recommend 6 cycles of R-CHOP-21 for fit older patients.
Selected studies of frontline treatment in older patients with DLBCL in the rituximab era
| Reference . | Treatment . | Age, median (range), y . | No. of patients . | PFS, % . | OS, % . |
|---|---|---|---|---|---|
| Delarue et al28 | 8 R-CHOP14 | 70 (60-80) | 304 | 60 (3 y) | 69 (3 y) |
| 8 R-CHOP21 | 70 (59-80) | 298 | 62 (3 y) | 72 (3 y) | |
| Peyrade et al6 | 6 R-miniCHOP | 83 (80-95) | 149 | 47 (2 y) | 59 (2 y) |
| Peyrade et al29 | 6 Ofatumumab-miniCHOP | 83 (80-95) | 120 | 57 (2 y) | 65 (2 y) |
| Merli et al23 | 6 Obinutuzumab-miniCHOP | 82 (68-89) | 34 | 49 (2 y) | 68 (2 y) |
| Oberic et al30 | 6 R-miniCHOP vs 6 R-miniCHOP + lenalidomide | 83 (80-96) | 127 | 56 (2 y) | 66 (2 y) |
| 122 | 55 (2 y) | 66 (2 y) | |||
| Moccia et al31 | 6-8 R-CEOP21 | 73 (34-93) | 70 | 53 (5 y) | 47 (5 y) |
| 6-8 R-CHOP21 | 73 (21-92) | 140 | 69 (5 y) | 65 (5 y) | |
| Shen et al32 | 6 R-GemOx | 60-69 | 14 | 71 (3 y) | 78 (3 y) |
| ≥70 | 46 | 42 (3 y) | 61 (3 y) | ||
| Fields et al33 | 6 R-GCVP | 76 (52-90) | 27 (LVEF ≤50%) | 55 (2 y) | 66 (2 y) |
| 35 (LVEF >50%) | 45 (2 y) | 46 (2 y) | |||
| Storti et al34 | 4-6 R-Benda + 2 R | 81 (71-89) | 45 | 38 (2 y) | 51 (2 y) |
| Reference . | Treatment . | Age, median (range), y . | No. of patients . | PFS, % . | OS, % . |
|---|---|---|---|---|---|
| Delarue et al28 | 8 R-CHOP14 | 70 (60-80) | 304 | 60 (3 y) | 69 (3 y) |
| 8 R-CHOP21 | 70 (59-80) | 298 | 62 (3 y) | 72 (3 y) | |
| Peyrade et al6 | 6 R-miniCHOP | 83 (80-95) | 149 | 47 (2 y) | 59 (2 y) |
| Peyrade et al29 | 6 Ofatumumab-miniCHOP | 83 (80-95) | 120 | 57 (2 y) | 65 (2 y) |
| Merli et al23 | 6 Obinutuzumab-miniCHOP | 82 (68-89) | 34 | 49 (2 y) | 68 (2 y) |
| Oberic et al30 | 6 R-miniCHOP vs 6 R-miniCHOP + lenalidomide | 83 (80-96) | 127 | 56 (2 y) | 66 (2 y) |
| 122 | 55 (2 y) | 66 (2 y) | |||
| Moccia et al31 | 6-8 R-CEOP21 | 73 (34-93) | 70 | 53 (5 y) | 47 (5 y) |
| 6-8 R-CHOP21 | 73 (21-92) | 140 | 69 (5 y) | 65 (5 y) | |
| Shen et al32 | 6 R-GemOx | 60-69 | 14 | 71 (3 y) | 78 (3 y) |
| ≥70 | 46 | 42 (3 y) | 61 (3 y) | ||
| Fields et al33 | 6 R-GCVP | 76 (52-90) | 27 (LVEF ≤50%) | 55 (2 y) | 66 (2 y) |
| 35 (LVEF >50%) | 45 (2 y) | 46 (2 y) | |||
| Storti et al34 | 4-6 R-Benda + 2 R | 81 (71-89) | 45 | 38 (2 y) | 51 (2 y) |
PFS, progression-free survival; R-CEOP, rituximab, cyclophosphamide, etoposide, vincristine and prednisone; R-Benda, rituximab and bendamustine; R, rituximab; R-GCVP, rituximab, gemcitabine, cyclophosphamide, vincristine and prednisone; R-GemOx, rituximab, gemcitabine and oxaliplatin.
Dose intensity
Several studies have shown that many older patients can be cured with R-CHOP and that the dose and dose intensity of chemotherapeutic agents being administered is important.3,42 It remains an open question as to what dose intensity is optimal in older patients in order to achieve the best treatment outcome. In patients with multiple comorbidities, higher dose intensities are likely associated with more toxicity and consequently, worse outcomes. In contrast, if the relative dose intensity decreases below 70%, the relapse rate increases dramatically.43 A recent systematic review assessed the impact of R-CHOP dose intensity on DLBCL survival outcomes, with a focus on the older. The study found evidence of improved survival with higher relative dose intensities (up to R-CHOP-21) in those aged <80 years, but the literature to date does not support full-dose intensity in those aged ≥80 years.44 The R-miniCHOP protocol is considered as the standard treatment in older patients aged ≥80 years in many parts of the world (Table 2).6
Prephase
Prephase treatment with corticosteroids can often improve the performance status of the patient and can potentially reduce the number of therapy-associated deaths, especially during the first cycle.29,37,45 A recent prospective, small pilot study investigated prephase treatment with rituximab and prednisone in patients with DLBCL aged ≥70 years or aged 60 to 70 years with a Karnofsky Performance Scale score of <80, treated with R-CHOP-21.46 The study showed that a senescence-associated proinflammatory cytokine milieu was readily reversed by prephase treatment.
We give 5 days of oral prednisone (100 mg) to most older patients before the first R-CHOP cycle. In combination with allopurinol and sufficient fluid intake, this will also decrease the incidence of tumor lysis syndrome. A prephase might not be necessary for patients with a good performance status and nonbulky disease. High doses of corticosteroids may increase the risk of insomnia, delirium, mood swings, and hyperglycemia, especially in patients aged >80 years. We advise to individualize the duration and the dose of the corticosteroids according to the patient’s performance status.
G-CSF prophylaxis
The risk of febrile neutropenia (FN) is increased in older patients. The FN risk is highest in the first cycle.47,48 Current guidelines recommend primary recombinant G-CSF prophylaxis in patients aged ≥65 years receiving R-CHOP.49,50 A large international observational study showed that, compared with younger patients, older patients receiving R-CHOP tended to be more likely to have other known risk factors for FN, including lower ECOG scores, more advanced Ann Arbor stage, elevated baseline lactate dehydrogenase levels, baseline hemoglobin values <12 g/dL, presence of comorbidities, and bone marrow involvement. Thus, the older population had several characteristics that made them particularly vulnerable to FN.47 The same study revealed that only 53% of older patients received primary prophylaxis despite meeting guideline criteria, suggesting underutilization of an important preventive measure. We recommend primary prophylaxis with G-CSF in all patients with DLBCL aged ≥65 years treated with curative intent with R-CHOP.
Supportive care
Rigorous supportive care during treatment is crucial in preventing severe side effects and fatal outcome. The incidence of grade 3 or 4 infections in patients treated with R-CHOP-21 is high. In large prospective studies, incidence rates of between 23% and 37% are reported.28,42 The German High-Grade Non-Hodgkin Lymphoma Study Group managed to reduce the treatment-related mortality due to severe infections with stringent infectious prophylaxis in older patients treated with R-CHOP-14 variants.51 Eyre et al52 assessed older patients who had received R-CHOP treatment for risk of infection-related hospital admissions and infection-related mortality. An intended dose intensity of >80% was associated with an increased risk of hospitalization owing to infection. They developed a predictive risk score for infection-related death, based on an IPI score of 3 to 5, a CIRS-G score ≥6, and low albumin. Primary quinolone prophylaxis independently reduced infection-related hospitalization but not infection-related death.52 Based on the absence of consensus guidelines, we do not recommend antimicrobial prophylaxis for all older patients, but it can be considered for patients at high risk. Only a randomized trial can provide evidence for the usefulness of antimicrobial prophylaxis. Immediate treatment of infections is essential. Patients anticipating treatment with rituximab-containing therapy should be tested for hepatitis B virus (HBV). Findings of chronic HBV or past HBV infection require HBV-activation risk assessment and these patients should be offered antiviral prophylaxis and/or careful monitoring.53
Some older patients experience severe fatigue between treatment cycles after stopping prednisone. These patients might benefit from hydrocortisone (20 mg in the morning, 10 mg in the afternoon). Another approach is to taper prednisone without increasing the overall exposure by replacing 100 mg on day 5 with 50 mg on day 5, 25 mg on day 6, and 12.5 mg on days 7 and 8.
Neurotoxicity is a dose-limiting side effect of vincristine. This may lead to severe peripheral sensory, motor, and autonomic neuropathies affecting quality of life and treatment delay. Symptoms include numbness, paresthesia, impaired balance, weakened tendon reflexes, and altered gait.54 Autonomic dysfunctions can cause constipation, paralytic ileus, urinary retention, and orthostatic hypotension.55,56 The severity of neuropathy increases with accumulated vincristine dose.57 Careful monitoring is important and, in older patients, the vincristine dosage might be reduced from the start to avoid complications. The prevalence of constipation appears to increase with increasing age, particularly after age 65.58 We often preemptively use laxatives, particularly in patients with chronic constipation.
In the individual older patient, toxicity during the first R-CHOP cannot easily be predicted. Therefore, we always see the patient between day 8 and 12 of R-CHOP to get a good indication of the individual’s tolerability of treatment.
CNS prophylaxis
CNS prophylaxis is advocated for patients with DLBCL at high risk of CNS relapse. There is a lack of robust evidence to clearly recommend which patients should receive CNS prophylaxis and how this should be delivered. Currently, strong evidence supporting the efficacy of intrathecal chemotherapy in preventing CNS relapse in DLBCL is lacking. Emerging data indicate that the efficacy of intravenous prophylaxis with high-dose methotrexate to prevent CNS relapse is doubtful.59-61 The CNS-IPI score can be used as a tool to estimate the risk of CNS relapse or progression.62 The CNS-IPI score incorporates all the original IPI features with the addition of kidney and/or adrenal involvement. One of the factors in the CNS-IPI score is age >60 years and therefore a significant proportion of older patients with DLBCL will fall into the high-risk category for CNS relapse. Therefore, in each individual older patient with DLBCL the potential gains of CNS prophylaxis should be carefully balanced against the risks of toxicities. Considering the emerging data, which bring into question the current practice of prophylactically administering intravenous or intrathecal methotrexate and the higher risk of toxicities, we often refrain from CNS prophylaxis in the older DLBCL population.
How do we treat an unfit older patient with DLBCL?
The current literature suffers from the scarcity of randomized trials studying alternative regimens in older patients with DLBCL. Most studies had a single-arm design. Table 2 provides a selection of mostly phase 2 studies. For an extensive overview of published studies, we refer to 2 review papers.63,64 Once a patient has been considered unfit, the assessment should be made whether the patient can tolerate therapy directed at cure. Although the FIL study by Tucci et al43 demonstrated that there are patients in the unfit group who do unequivocally benefit from full-dose curative intent treatment, the study by Merli et al23 in a larger cohort demonstrated that dose reductions are not much more inferior than full-dose anthracycline in unfit patients and can enable cure, albeit lower than with full dose, while limiting toxicity.
Besides a clinical geriatric assessment, the decision on anthracycline dose reduction or replacement also depends on cardiac status. Risk factors for anthracycline-related cardiotoxicity include lifetime cumulative dose, infusion regimen, and any condition that increases cardiac susceptibility, including preexisting cardiac disease, hypertension, concomitant use of other chemotherapies or mediastinal radiation therapy, and older age (>65 years).65,66 In patients with 1 or multiple risk factors for anthracycline-related cardiotoxicity, the cumulative dose vs cardiotoxicity curve is shifted to the left and these patients are candidates for cardiovascular profiling and risk stratification before treatment, and should be monitored carefully.67 In our hospital we refer these patients to the oncocardiology unit of the department of cardiology. In patients without objective cardiac impairment, normal LVEF, and without additional risk factors, standard dose doxorubicin can be considered with close monitoring. If full-dose R-CHOP proves to be too toxic besides cardiac toxicity, dose reduction to R-miniCHOP can be implemented as discussed earlier. If during treatment, signs of clinically significant cardiac toxicity is noted there are reasonable alternatives such as replacement of doxorubicin by etoposide (50 mg/m2 intravenously on day 1 and 100 mg/m2 orally on days 2 and 3, R-CEOP, Table 2).31
To date, only 1 randomized phase 3 study has been performed in patients aged ≥80 years and it failed to show a benefit from the addition of lenalidomide to R-miniCHOP. However, the study did show that a large prospective study is feasible in this patient population.30
Several other alternatives for the use of anthracyclines have been studied in small series. R-GemOx has been studied in a frontline setting for patient with DLBCL aged ≥70 years, or aged 60 to 69 years with performance status 2, and for patients with and IPI score of 0 to 2 and this yielded satisfactory response rates with a 2-year OS of 85%. This small study of 60 patients provides evidence that when anthracyclines are contraindicated, this regimen could be a feasible alternative (Table 2).32 Replacement of doxorubicin by gemcitabine in R-CHOP has been studied in the frontline setting in patients with impaired LVEF.33 Response rates were satisfactory for patients receiving all 6 cycles but almost half of the patients did not finish treatment owing to adverse events. The overall response for the whole group was 61.3% (95% CI, 49.2-73.4) and the 2-year OS was 55.8% (95% CI, 43.3-68.4) rendering this a fairly acceptable alternative to R-CHOP. The response to rituximab-bendamustine is disappointing. Although overall response rates are fairly good, the 2-year PFS was only 38%, rendering this regimen not well suited, despite excellent tolerability.34
An interesting trial, investigating the addition of oral azacitidine to R-miniCHOP in patients aged >75 years, is currently ongoing by the Southwest Oncology Group.68 The rationale behind that study was that increased tumor methylation is a biologic feature of older patients. The trial incorporates the FIL tool for frailty assessment and a serial CGA to evaluate effects of therapy on functional status over time.
Therefore, when treating unfit older patients, we generally advise R-miniCHOP with the option to escalate to full dose if well tolerated. A common practice is to use a dynamic dosing strategy in which the doses of doxorubicin and cyclophosphamide are escalated if the previous cycle was well tolerated without toxicities, and to deescalate in case of toxicities. If anthracyclines are contraindicated, we usually replace the anthracyclines with etoposide with the option of giving R-miniCEOP when dose reductions are indicated.
How do we treat a frail older patient with DLBCL?
Frail older patients are those who cannot tolerate dose-modified regimens such as R-miniCHOP and consequently these patients cannot be cured with currently available regimens.
Further dose reductions are of no benefit and will likely only harm patients and hinder good palliative care. Radiotherapy can obviously be used for localized disease and/or palliation of symptomatic disease, and sometimes the use of corticosteroids can have temporary benefit.
In individual cases, in which frailty is suspected to mostly be due to age and the untreated disease rather than comorbidity, disease control can sometimes result in rapid improvement of performance status. In such patients, an attenuated strategy could be beneficial.
However, the development of bispecific antibodies may change the outlook of these patients.69-71 These bispecific antibodies redirect T cells to eliminate malignant B cells by binding to CD3 on T cells and CD20 on B cells. Early data from an ongoing phase-1/2 trial (NCT02500407) demonstrated that in a cohort of unfit older patients, mosunetuzumab monotherapy was well tolerated and had encouraging response rates with a complete remission in 8 of 19 patients.69,71 The most common adverse event seen in bispecific antibodies is cytokine release syndrome, occurring in 27%-59% of patients, mainly of grade 1 or 2 severity and confined to the first cycle. Although 19%-25% of patients experienced grade ≥3 neutropenia, the reported incidence of febrile neutropenia is low.70-72 Several trials are currently exploring the use of these bispecific antibodies as monotherapy or in combination with other drugs.
Conclusions and future directions
DLBCL is most common in the older population and whereas most fit patients will tolerate standard treatments, special attention to age-dependent comorbidity is warranted to guide treatment. Geriatric assessments can be helpful to distinguish fit, unfit, and frail patients and to identify those who are most likely to benefit from curative intent treatment and in whom a mitigated initial approach is warranted. The EPI was specifically designed for older patients with aggressive lymphoma. We recommend referral to a cardiologist in patients with an increased risk of cardiac toxicity. The patients in both case presentations were seen with regular intervals in the oncocardiology unit before, during, and after the treatment. A short prephase with corticosteroids can improve the performance status. Judicious use of supportive care, such as primary G-CSF prophylaxis, is crucial to prevent a fatal outcome when treating older patients with chemo-immunotherapy. For unfit patients and the very older, a dose-reduction regimen, such as R-miniCHOP is feasible. When anthracyclines are contraindicated, doxorubicin can be replaced by etoposide (R-CEOP) or gemcitabine. Patients who are frail most often do not benefit from chemotherapy. In selected cases, in which frailty is mostly due to symptomatic disease, chemotherapy may resolve frailty.
There is an unmet need for noncytotoxic therapies in older patients. New antibodies, bispecific antibodies, antibody-drug conjugates, and immunomodulatory agents have toxicity profiles that differ from that of conventional chemotherapeutics. These drugs are generally well tolerated. Several studies are ongoing with these agents, either as monotherapy or in combination. In order to improve our knowledge of the optimal treatment of older patients with DLBCL, studies should have eligibility criteria that reflect the real-world patient population. Studies should also incorporate patient-centered end points and be powered to detect significant differences in these outcomes.
Acknowledgment
The authors thank Jeanette Doorduijn, hematologist, for her critical review of the final version of the manuscript.
Authorship
Contribution: P.J.L. conceived, wrote, and reviewed the paper; and P.G.N.J.M. wrote and reviewed the paper.
Conflict-of-interest disclosure: P.J.L. received research grants from Takeda, Servier, and Roche, and honoraria for advisory boards from Takeda, Servier, Roche, Celgene, Genmab, AbbVie, Incyte, and Regeneron. P.G.N.J.M. received a research grant from AstraZeneca and honoraria for advisory boards from Bristol Myers Squibb and GlaxoSmithKline.
Correspondence: Pieternella J. Lugtenburg, Department of Hematology, Erasmus MC Cancer Institute, University Medical Center, Dr Molewaterplein 40, 3015 GD Rotterdam, The Netherlands; e-mail: p.lugtenburg@erasmusmc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal