TO THE EDITOR:
Thrombotic thrombocytopenic purpura (TTP) is a rare life-threatening thrombotic microangiopathy characterized by nonimmune hemolytic anemia, thrombocytopenia, and central nervous system abnormalities1,2; however, multiorgan involvement and renal dysfunction1-4 may occur. The disease is due to the deficiency of a plasma metalloprotease named ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) that cleaves ultralarge von Willebrand factor (VWF) multimers.2 In TTP, the excess of ultralarge multimers of VWF, which are not cleaved anymore by ADAMTS13, substains a thrombotic condition via endothelial injury and intraluminal platelets thrombosis, causing platelet consumption, organ damages, and mechanical hemolysis.5 The defect of ADAMTS13, in the majority of cases, is acquired because of the development of anti-ADAMTS13 autoantibodies (immune-mediated TTP [iTTP])6 or, in very few cases, may be genetically determined as autosomal recessive disorder caused by ADAMTS13 mutations.7 In TTP, although the pathogenic role of ultralarge VWF multimers is well established,5 increasing evidence indicates that complement involvement can also occur.8-11 The complement system is an important part of innate immunity, organized in 3 enzymatic cascades that converge toward a common pathway with the formation of strong inflammatory mediators (C3a/C5a) and the production of the terminal complex (C5b-9) that lyses target cells. The system is finely regulated by soluble and cell-bound inhibitors.12,13 When the complement is overactivated, host tissue injury can occur, with the kidney being the main target organ. Indeed, complement activation is frequently observed in several renal diseases14 and has been recognized as a critical mediator in their development and progression. In particular, in atypical hemolytic uremic syndrome (aHUS), another thrombotic microangiopathy in which kidney injury is a characteristic, the complement system is hyperactivated because of genetic or acquired disorders of its regulatory components.15 In the pathophysiology of TTP, complement activation may play an additional role and has been associated with increased mortality.9 To the best of our knowledge, in acquired TTP, the association of complement activation with renal dysfunction has never been assessed.
With this background, we evaluated our case list of patients with iTTP, searching for signs of complement activation and their possible associations with different clinical conditions, in particular renal dysfunction.
We studied 58 consecutive cases of patients with iTTP during acute phase (42 women and 16 men), with a median age of 45 years (range, 18-79 years). Demographic and clinical characteristics of the study population are reported in Table 1. The diagnosis of iTTP was defined by the presence of thrombocytopenia and microangiopathic hemolytic anemia, confirmed by the severe deficiency of ADAMTS13 (activity < 10% of normal level) and the presence of anti-ADAMTS13 autoantibodies. Samples were collected at acute iTTP presentation, before any plasma therapy. A total of 24 healthy participants served as healthy controls (17 women and 7 men; median age, 44 years; range, 20-76 years). A positive control group consisted of 46 patients with aHUS (33 women and 13 men; median age, 37 years; range, 16-80 years). The study was approved by the ethics committee of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico (no. 123/18-12-2013) and was carried out in conformity with the 2013 revision of the Declaration of Helsinki and the code of good clinical practice. All participants gave their written informed consent.
Demographic and clinical characteristics of 58 patients with acquired TTP
| Demographic characteristics . | Acquired TTP (n = 58) . | Normal values . |
|---|---|---|
| Male/female | 14/44 | |
| Age, median (range), y | 45 (18-79) | |
| Age at first episode, median (range), y | 43 (18-79) | |
| Clinical features at acute presentation, n (%)∗ | ||
| First TTP event | 42 (72%) | |
| Systemic symptoms | 45 (78%) | |
| Neurological alterations | 27 (47%) | |
| Cardiac alterations | 8 (14%) | |
| Renal alterations | 13 (22%) | |
| Bleeding | 41 (71%) | |
| Laboratory features at acute presentation, median (range) | ||
| Platelet count, 109/L | 16 (4-106) | 150-450 |
| Hb, g/dL | 8.8 (5.2-14.8) | 13.0-16.0 |
| LDH, IU/L | 1136 (278-7243) | 105-333 |
| Creatinine, mg/dL | 0.90 (0.40-6.20) | 0.60-1.20 |
| Complement activation in different clinical conditions, median (range), sC5b-9, ng/mL | ||
| All conditions, n = 58 | 351 (78-1106)∗ | 120 (68-202) |
| Systemic symptoms, n = 45 | 365 (124-1106) | |
| No systemic symptoms, n = 13 | 330 (78-660) | |
| Neurological alterations, n = 31 | 339 (124-1051) | |
| No neurological alterations, n = 27 | 338 (78-1106) | |
| Cardiac alterations, n = 8 | 384 (253-1051) | |
| No cardiac alterations, n = 50 | 345 (78-1106) | |
| Renal alterations, n = 13 | 460 (221-1051)† | |
| No renal alterations, n = 45 | 322 (78-1106) | |
| Bleeding, n = 41 | 331 (78-1106) | |
| No bleeding, n = 17 | 381 (123-856) |
| Demographic characteristics . | Acquired TTP (n = 58) . | Normal values . |
|---|---|---|
| Male/female | 14/44 | |
| Age, median (range), y | 45 (18-79) | |
| Age at first episode, median (range), y | 43 (18-79) | |
| Clinical features at acute presentation, n (%)∗ | ||
| First TTP event | 42 (72%) | |
| Systemic symptoms | 45 (78%) | |
| Neurological alterations | 27 (47%) | |
| Cardiac alterations | 8 (14%) | |
| Renal alterations | 13 (22%) | |
| Bleeding | 41 (71%) | |
| Laboratory features at acute presentation, median (range) | ||
| Platelet count, 109/L | 16 (4-106) | 150-450 |
| Hb, g/dL | 8.8 (5.2-14.8) | 13.0-16.0 |
| LDH, IU/L | 1136 (278-7243) | 105-333 |
| Creatinine, mg/dL | 0.90 (0.40-6.20) | 0.60-1.20 |
| Complement activation in different clinical conditions, median (range), sC5b-9, ng/mL | ||
| All conditions, n = 58 | 351 (78-1106)∗ | 120 (68-202) |
| Systemic symptoms, n = 45 | 365 (124-1106) | |
| No systemic symptoms, n = 13 | 330 (78-660) | |
| Neurological alterations, n = 31 | 339 (124-1051) | |
| No neurological alterations, n = 27 | 338 (78-1106) | |
| Cardiac alterations, n = 8 | 384 (253-1051) | |
| No cardiac alterations, n = 50 | 345 (78-1106) | |
| Renal alterations, n = 13 | 460 (221-1051)† | |
| No renal alterations, n = 45 | 322 (78-1106) | |
| Bleeding, n = 41 | 331 (78-1106) | |
| No bleeding, n = 17 | 381 (123-856) |
Clinical signs and symptoms were categorized as follows: systemic (fatigue, fever, abdominal pain, headache, jaundice, and vomiting), neurological (stroke, seizures, coma, personality change, focal neurological signs, and transitory ischemic attack), cardiac (acute coronary syndrome and electrocardiographic ischemic abnormalities), renal (an increase in serum creatinine ≥0.3 mg/dL within 48 hours, ≥50% within 7 days, or a urine output of <0.5 mL/kg per hour for >6 hours, per the Kidney Disease Improving Global Outcomes guidelines), and bleeding (hematuria, meno-metrorrhagia, mucosal bleeding, gastrointestinal tract bleeding, ecchymosis, and purpura).
Hb, hemoglobin; LDH, lactate dehydrogenase.
sC5b-9 in all the conditions vs healthy controls P = .0001.
sC5b-9 in renal alterations vs no renal alterations P = .01.
ADAMTS13 activity plasma levels were measured with a fluorescence resonance energy transfer assay that uses a synthetic 73–amino acid peptide (FRETS-VWF73), as previously described.16 Intra- and interassay coefficients of variation (CVs) were 6% and 9.5%, respectively.
Antibodies to ADAMTS13 were measured in the plasma using an in-house enzyme-linked immunosorbent assay (ELISA), as previously described.17 Intra- and interassay CVs were lower than 15%.
Soluble C5b-9 (sC5b-9) complex plasma levels were determined using a commercial ELISA kit (MicroVue Complement SC5b-9 Plus EIA, Quidel, San Diego, CA). Intra- and interassay CVs were 6.8% and 13.1%, respectively.
Complement genetic studies were performed to rule out aHUS among the 13 patients with TTP with renal impairment via next-generation sequencing and multiplex ligation–dependent probe amplification, as previously described.18 In particular, we filtered variants using a minor allele frequency of <0.03 (1000 Genome Phase 3 and gnomAD databases). Then we applied a functional effect filter considering missense, splicing, stop gained, and frameshift variants. Missense variants with a possible damaging effect based on several prediction tools were included.
A Mann-Whitney U test was used to compare patients with TTP and aHUS vs controls, whereas Wilcoxon-signed rank test was used to compare patients with TTP in acute vs remission phase. To evaluate the association of sC5b-9 in acute phase with continuous variables, we fitted univariate and multivariable linear regression models. Continuous variables except hemoglobin were log transformed using natural logarithms to achieve normal distributions. Regression estimates were expressed as percent change (and 95% confidence intervals [CIs]) per 1 logarithmic increase of the independent variable (sympercent = slope × 100).19 For dichotomous end points, we fitted the univariate and multivariable logistic models and calculated odds ratios (ORs) and CIs. Multivariable models were adjusted for sex, age (continuous), and type of episode (first TTP event vs relapse). Data were analyzed using SPSS version 27 (IBM SPSS Inc, Chicago, IL) and Stata 17 (StataCorp 2021, College Station, TX).
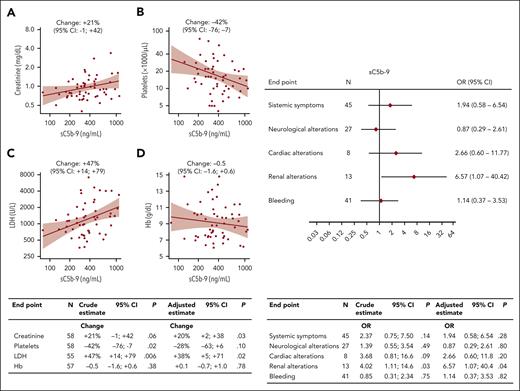
All patients with iTTP during acute phase had plasma levels of ADAMTS13 antigen and activity <10% and were positive for autoantibodies to ADAMTS13 with a median of 59 AU/mL (range, 3-1050 AU/mL). Plasma levels of sC5b-9, reliable marker of complement activation, were higher (P = .0001) in acute patients (median, 351 ng/mL; range, 78-1106 ng/mL) than in healthy controls (median, 120 ng/mL; range, 68-202 ng/mL) (Table 1). Supplemental Figure 1, available on the Blood website, shows sC5b-9 levels in patients with acute TTP or aHUS, with the latter of these being 2.3-fold higher (P = .0001). In patients with TTP during remission20, sC5b-9 levels were lower than it was during the acute phase (P = .0001; supplemental Figure 2) but remained higher (median, 251 ng/mL; range, 99-713 ng/mL) than that in controls (P = .0001). During remission, no significant correlation was observed between plasma levels of sC5b-9 and ADAMTS13 plasma activity. Eighty-five percent of acute patients had sC5b-9 levels above the upper limit of controls. Moreover, sC5b-9 levels during acute phase were positively correlated with levels of creatinine (marker of renal dysfunction) and lactate dehydrogenase (marker of hemolysis and cell damage), both in univariate and multivariable linear regressions, and inversely correlated with platelet number (Figure 1, left). In our patients with iTTP, plasma sC5b-9 was not significantly associated with systemic symptoms, neurological alterations, cardiac alterations, and bleeding (Table 1; Figure 1, right). In contrast, the 13 patients with renal impairment had higher plasma levels of sC5b-9 (460 ng/mL) than patients with healthy renal function (322 ng/mL; P = .01) (Table 1), corresponding to a crude OR of 4.02 (95% CI, 1.11-14.6) per 1 ln(sC5b-9) increase. In the multivariable logistic regression model, this strong positive association between complement activation and renal alterations was confirmed (adjusted OR, 6.57; 95% CI, 1.07-14.4) (Figure 1, right). The same holds true when the analysis was restricted to the 42 first TTP events, which were notoriously more severe than relapse events (OR, 7.10; 95% CI, 0.98-51.6). None of the patients with renal alterations had a known chronic kidney disease before TTP, and in all patients, the renal alterations recovered within the subsequent 6 months. All of our 58 patients with acute TTP survived and recovered from the episode. Variants in complement regulatory genes were present in 9 of the 13 patients with TTP, renal dysfunction, and elevated complement activation (supplemental Table 1), and none of the patients had anti–factor H autoantibodies, when tested with ELISA.18
Association of sC5b-9 plasma levels with laboratory parameters and with clinical conditions in 58 patients with TTP during acute phase. Linear regression analyses (left) showing association with creatinine (A), platelets (B), LDH (C), and Hb (D) are shown. For ln-transformed continuous end points (creatinine, platelets, and LDH), the estimate should be interpreted as percent change per 1 ln(sC5b-9) increase; for Hb the change is in g/dL. Continuous lines in the graphs represent predicted lines from crude linear regression models, shaded areas represent 95% confidence bands. In the table (bottom), each end point reports the number of observation (N), the percent changes per 1 ln(sC5b-9) (crude estimate from univariate analysis and adjusted estimate from multivariable analysis), the 95% CIs, and the P value (P). Multivariable models were adjusted for sex, age (continuous), and type of episode (first TTP event vs relapse). Logistic regression analyses (right) showing ORs and 95% CIs per 1 ln(sC5b-9) increase, for systemic symptoms (fatigue, fever, abdominal pain, headache, jaundice, and vomiting), neurological alterations (stroke, seizures, coma, personality change, focal neurological signs, and transitory ischemic attack), cardiac alterations (acute coronary syndrome and electrocardiographic ischemic abnormalities), renal alterations (an increase in serum creatinine ≥0.3 mg/dL within 48 hours, ≥50% within 7 days, or a urine output of <0.5 mL/kg per hour for >6 hours, per the Kidney Disease Improving Global Outcomes guidelines), and bleeding (hematuria, meno-metrorrhagia, mucosal bleeding, gastrointestinal tract bleeding, ecchymosis, and purpura) are shown. In the table (bottom), each end point reports the number of patients (N), the percent changes per 1 ln(sC5b-9) (crude estimate from univariate analysis and adjusted estimate from multivariable analysis), the 95% CI, and the P value. Multivariable models were adjusted for sex, age (continuous), and type of episode (first TTP event vs relapse).
Association of sC5b-9 plasma levels with laboratory parameters and with clinical conditions in 58 patients with TTP during acute phase. Linear regression analyses (left) showing association with creatinine (A), platelets (B), LDH (C), and Hb (D) are shown. For ln-transformed continuous end points (creatinine, platelets, and LDH), the estimate should be interpreted as percent change per 1 ln(sC5b-9) increase; for Hb the change is in g/dL. Continuous lines in the graphs represent predicted lines from crude linear regression models, shaded areas represent 95% confidence bands. In the table (bottom), each end point reports the number of observation (N), the percent changes per 1 ln(sC5b-9) (crude estimate from univariate analysis and adjusted estimate from multivariable analysis), the 95% CIs, and the P value (P). Multivariable models were adjusted for sex, age (continuous), and type of episode (first TTP event vs relapse). Logistic regression analyses (right) showing ORs and 95% CIs per 1 ln(sC5b-9) increase, for systemic symptoms (fatigue, fever, abdominal pain, headache, jaundice, and vomiting), neurological alterations (stroke, seizures, coma, personality change, focal neurological signs, and transitory ischemic attack), cardiac alterations (acute coronary syndrome and electrocardiographic ischemic abnormalities), renal alterations (an increase in serum creatinine ≥0.3 mg/dL within 48 hours, ≥50% within 7 days, or a urine output of <0.5 mL/kg per hour for >6 hours, per the Kidney Disease Improving Global Outcomes guidelines), and bleeding (hematuria, meno-metrorrhagia, mucosal bleeding, gastrointestinal tract bleeding, ecchymosis, and purpura) are shown. In the table (bottom), each end point reports the number of patients (N), the percent changes per 1 ln(sC5b-9) (crude estimate from univariate analysis and adjusted estimate from multivariable analysis), the 95% CI, and the P value. Multivariable models were adjusted for sex, age (continuous), and type of episode (first TTP event vs relapse).
Our data support the view that the complement system may be involved in the pathophysiology of the disease. Indeed, the terminal complex of complement may activate endothelial cells, neutrophils, and platelets,21-23 thus, concurring with ultralarge multimers of VWF to the formation of microthrombi and the subsequent hemolytic anemia. However, uncleaved ultralarge VWF strings secreted by and anchored to endothelial cells may per se trigger the complement system as demonstrated in cultured human umbilical vein endothelial cells.24 The synergistic effects of ADAMTS13 deficiency and complement activation in the pathogenesis of thrombotic microangiopathy has also been supported by experimental data showing that mice carrying both ADAMTS13 homozygous deficiency and homozygous deficiency of the complement inhibitor factor H exhibit a severe thrombotic microangiopathy.25 In our patients with TTP with renal impairment, the variants of complement regulatory genes, some of which are considered benign in the general population, may have affected the endothelial susceptibility to injury in the presence of ultralarge VWF multimers. A similar explanation was used by Jodele et al26 who proposed, in patients with hematopoietic stem cell transplantation, that complement gene variants may modify the susceptibility to thrombotic microangiopathy after stressors on endothelium, such as chemotherapy, calcineurin inhibitors, or graft-versus-host disease. The involvement of the kidney microcirculation in TTP may be milder and more delayed than in aHUS owing to lower increase in complement activation. All these aspects warrant further investigation, in particular with the full genetic study of patients with and without complement activation and renal impairment.
In conclusion, our data show that in acquired TTP, the complement system is frequently activated with higher levels of activation during the acute phase of the disease, particularly in patients with renal alterations, in which the highest levels of complement activation are associated with variants in complement regulatory genes. The direct correlation between complement activation and renal dysfunction suggests the role of complement in kidney microvascular damage and provide the rationale for proposing studies aimed to evaluate the use of anticomplement drugs in patients with acute TTP and renal involvement.
Acknowledgments
This work was partially supported by the Italian Ministry of Health – Bando Ricerca Corrente 2022. The Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico is a member of the European Reference Network (ERN) EuroBloodNet.
Authorship
Contribution: M.C. designed the study in collaboration with F.P. and wrote the manuscript; F.P., B.F., A.A., and G.A. were responsible for the clinical management of the patients; I.M., S.G., E.G., and L.P. were responsible for the laboratory tests; V.D.Z. helped in organizing the data; D.C. performed the statistical analysis; and all authors contributed to the interpretation of the results, critically reviewed the manuscript, and approved the final version for submission.
Conflict-of-interest disclosure: I.M. received honoraria for participating as a speaker at educational meetings organized by Instrumentation Laboratory and Sanofi. G.A. is a member of the scientific advisory board of the Global Atypical Hemolytic Uremic Syndrome Registry supported by Alexion Pharmaceuticals Inc, and he has received honoraria from Alnylam, Roche, Novartis, and Alexion for his participation in scientific advisory boards or for giving lectures. B.F. and A.A. received honoraria for participating as speakers at educational meetings organized by Sanofi. F.P. has received honoraria for participating as a speaker in education meetings organized by Grifols and Roche and she is a member of scientific advisory boards of BioMarin, Roche, Sanofi, Sobi, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Massimo Cugno, Department of Pathophysiology and Transplantation, Università degli Studi di Milano, Via Francesco Sforza 35, 20122 Milan, Italy; e-mail: massimo.cugno@unimi.it.
References
Author notes
Data are available on request from the corresponding author, Massimo Cugno (massimo.cugno@unimi.it).
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal