Abstract
Classic Hodgkin lymphoma (cHL) is characterized by a tumor microenvironment (TME) containing inflammatory/immune cells. Follicular lymphoma, mediastinal gray zone lymphoma, and diffuse large B-cell lymphomas may show a TME containing inflammatory/immune cells, but the TMEs are quite different. In B-cell lymphomas and cHL, programmed cell death 1 (PD-1)–PD ligand 1 pathway blockade drugs differ in their effectiveness among patients with refractory/relapsed disease. Further research should explore innovative assays that could reveal which molecules influence sensitivity or resistance to therapy in an individual patient.
Introduction
Hodgkin lymphoma (HL) is officially classified as classic HL (cHL) and nodular lymphocyte predominant HL (NLPHL).1,2 The term nodular lymphocyte predominant B-cell lymphoma has been recently proposed for NLPHL because of its close relationship to T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL).3 Classic HL is a distinct lymphoma characterized by pathognomonic tumor cells, the so called Hodgkin Reed-Sternberg (HRS) cells, which might be associated with Epstein Barr virus infection.4 HRS cells are typically surrounded by large numbers of immune and inflammatory cells.5 Tumor microenvironment (TME) has been used and still is used as a basis for defining mixed cellularity cHL, nodular sclerosis cHL (NScHL), and lymphocyte depletion cHL subtypes.6-8
HRS cells grow and survive through cross talk with immune and inflammatory cells of the TME,5,9,10 and escape immunosurveillance through a mechanism that regulates T-cell-mediated immune responses.11 HRS cells with surrounding T cells participate in the programmed cell death ligand 1 (PD-L1)--programmed cell death (PD)-1 pathway, which functions as a checkpoint that inactivates tumor-specific T cells. Concordantly, checkpoint-inhibiting antibodies have shown good results in the treatment of relapsed or refractory cHL.12-15 In the cHL model, other components of the TME have been demonstrated as prognostic/predictive factors. The abundance of tumor-associated macrophages (TAMs) is predictive of the resistance to either primary treatment with doxorubicin, bleomycin, vinblastine, and dacarbazine or salvage therapy with autologous stem cell transplant (ASCT).16
B-cell lymphomas other than cHL may show an inflammatory TME mirroring that of cHL.11 In certain cases, including mediastinal B-cell lymphomas and, occasionally, in diffuse large B-cell lymphomas (DLBCLs) and follicular lymphomas (FLs), a fraction of patients benefited from immunotherapy and checkpoint inhibitor treatments likely because of the overexpression of the PD-L1/PD-1 axis.11,17-22 In this article, we discuss the use of the TME cellularity and its spatial organization for stratifying patients affected by lymphoma to be subjected to immunotherapy and specifically checkpoint inhibitor treatments.
TME in cHL and other B-cell lymphomas
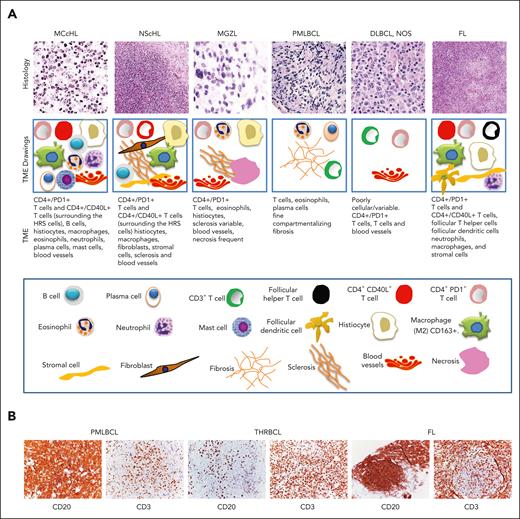
The TME in cHL is composed of several cell types and is different in each subtype of the disease.23 In mixed cellularity cHL, the TME includes a polymorphous cellularity with B cells and T cells, neutrophils, eosinophils, macrophages, plasma cells, and mast cells.5 The TME in NScHL demonstrates a characteristic involvement of fibroblast-like cells and sclerosis (Figure 1A). The TME in lymphocyte depletion cHL, a rare subtype, is characterized by many macrophages, few lymphocytes and histiocytes, and irregular fibrosis.29 The TME in lymphocyte rich cHL, which is equally rare, is rich in B cells and histiocytes.30
Tumor microenvironment in Hodgkin lymphoma and other B-cell lymphomas. (A) Histology and morphological characteristics of TME of cHL, MGZL, PMLBCL, DLBCL not otherwise specified and FL. Histology images (top row) showing the different subtypes of lymphomas and the corresponding drawings (second row) showing the cell types in their TMEs. As shown in the drawings, the TME in cHL demonstrates variable cellularity, which is different in each subtype. In mixed cellularity cHL, the TME consists of a polymorphous reactive infiltrate of B and T cells, neutrophils, histiocytes, plasma cells, and mast cells. In NScHL, the TME is specifically characterized by fibroblast-like cells and sclerosis. In MGZL, the TME typically comprises histiocytes, irregular sclerosis, and necrosis. In PMLBCL, the TME is variable but usually consists of histiocytes, lymphocytes, and compartmentalizing fibrosis. In DLBCL not otherwise specified (NOS), the TME is diminished but is quite variable; In FLs, the TME is partly similar to that of cHL, although in FL, it is rich in follicular dendritic cells. (third row) the cell types shown in the drawings, and (bottom row) the keys of the cell types. (B) The composite figure shows CD20 and CD3 immunostaining in 2 specific DLBCLs, PMLBCL and T-cell/histiocyte-rich B-cell lymphoma, and FL. In all of these lymphomas, tumor cells are immunostained for CD20, whereas their TME contains reactive infiltrates rich in CD3+ T cells. Topological and quantitative analyses of microenvironmental cells interacting with tumor cells were performed using an unsupervised clustering analysis of the TME. This analysis revealed that the TME in THRLBCL is distinct from cHL and DLBCL NOS with respect to PD-1/PD-L1 expression and spatial organization (spatial immune signature).24 In FLs and DLBCL NOS, which are often resistant to anti–PD-1 therapy,25,26 PD-L1 copy gains are not observed.27,28 It has been speculated that the lower incidence of tumor cell-specific PD-L1 expression in FL and DLBCL NOS, when compared with that in cHL, is compensated for by the higher density of PD-L1+ TAMs to ensure adequate PD-1 engagement for immune evasion.24 Original magnification ×10 (NScHL, FL), ×20 (PMLBCL, DLBCL NOS), and ×40 (MCcHL, GZL) (A) and ×20 (B).
Tumor microenvironment in Hodgkin lymphoma and other B-cell lymphomas. (A) Histology and morphological characteristics of TME of cHL, MGZL, PMLBCL, DLBCL not otherwise specified and FL. Histology images (top row) showing the different subtypes of lymphomas and the corresponding drawings (second row) showing the cell types in their TMEs. As shown in the drawings, the TME in cHL demonstrates variable cellularity, which is different in each subtype. In mixed cellularity cHL, the TME consists of a polymorphous reactive infiltrate of B and T cells, neutrophils, histiocytes, plasma cells, and mast cells. In NScHL, the TME is specifically characterized by fibroblast-like cells and sclerosis. In MGZL, the TME typically comprises histiocytes, irregular sclerosis, and necrosis. In PMLBCL, the TME is variable but usually consists of histiocytes, lymphocytes, and compartmentalizing fibrosis. In DLBCL not otherwise specified (NOS), the TME is diminished but is quite variable; In FLs, the TME is partly similar to that of cHL, although in FL, it is rich in follicular dendritic cells. (third row) the cell types shown in the drawings, and (bottom row) the keys of the cell types. (B) The composite figure shows CD20 and CD3 immunostaining in 2 specific DLBCLs, PMLBCL and T-cell/histiocyte-rich B-cell lymphoma, and FL. In all of these lymphomas, tumor cells are immunostained for CD20, whereas their TME contains reactive infiltrates rich in CD3+ T cells. Topological and quantitative analyses of microenvironmental cells interacting with tumor cells were performed using an unsupervised clustering analysis of the TME. This analysis revealed that the TME in THRLBCL is distinct from cHL and DLBCL NOS with respect to PD-1/PD-L1 expression and spatial organization (spatial immune signature).24 In FLs and DLBCL NOS, which are often resistant to anti–PD-1 therapy,25,26 PD-L1 copy gains are not observed.27,28 It has been speculated that the lower incidence of tumor cell-specific PD-L1 expression in FL and DLBCL NOS, when compared with that in cHL, is compensated for by the higher density of PD-L1+ TAMs to ensure adequate PD-1 engagement for immune evasion.24 Original magnification ×10 (NScHL, FL), ×20 (PMLBCL, DLBCL NOS), and ×40 (MCcHL, GZL) (A) and ×20 (B).
NScHL and primary mediastinal large B-cell lymphomas (PMLBCLs), a specific lymphoma of large B cells31,32 (Figure 1A-B), exhibit overlaps in pathologic, genetic, and molecular features, both between them and with the so called mediastinal gray zone lymphoma (MGZL), previously designated as B-cell lymphoma unclassifiable.3,33,34 The TME of MGZL is similar to that of NScHL and contains sparse inflammatory infiltrates with eosinophils, plasma cells, histiocytes, and T cells. Compared with NScHL, the TME of PMLBCL has a diminished background and contains eosinophils and T cells. Moreover, in the MGZL the sclerosis is variable, whereas in PMLBCL, a fine compartmentalizing fibrosis is usually observed. Necrosis is usually frequent in MGZL34,35 (Figure 1A). The TME in DLBCL, not otherwise specified encompasses all cases of large B-cell lymphoma that do not belong to a specific category3 and is quite variable and may not be easily described with 1 characteristic (Figure 1A). The TME of THRLBCL, another specific lymphoma of large B cell32,36 (Figure 1B), though resembles morphologically the TME of cHL differs from it in the higher densities of TAMs and PD-1 T cells.24
FL differs from other lymphomas by growing in a nodular structure, probably because of the lymphoma-TME interaction.37 The TME in FL includes immunocompetent lymphoid cells, stromal cells, and components of the extracellular matrix (Figure 1A). The cellular interactions in the TME are similar to those of normal germinal center B cells in the follicular microenvironment during normal immune reactions38 (Figure 1B).
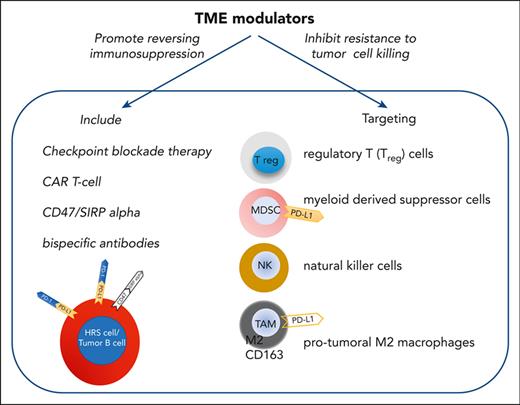
Therefore, among B-cell lymphomas, PMLBCL, MGZL, and THRLBCL exhibit a TME similar to that of mixed cellularity cHL or NScHL and potentially benefit from TME modulators. They are capable of targeting a variety of cell types, such as regulatory T cells, myeloid-derived suppressor cells, natural killer (NK) cells, and protumoral M2 macrophages, which promote immune suppression and immune exhaustion, making it difficult for tumor effector cells to kill malignant cells. By reversing immunosuppression and dysfunction, TME modulators, including checkpoint inhibitors, bispecific antibodies, and chimeric antigen receptor T cells might play a key role in the development of effective immunotherapeutic approaches.11,39-42
Functional interaction between tumor cells and their cellular microenvironment
An extensive cross talk occurs between tumor cells of B-cell lymphomas and immune cells of the TME (Figure 2), as observed in cHL.1 In the latter, this cross talk is mediated either by a large network of cytokines and chemokines expressed by HRS cells or molecules produced by different cell types of the TME,5 as has been reported for CD30/CD30L, CD40/CD40L, OX40L/OX40, Il-3/Il-3R, CCR5/CCL5, CD74 macrophage migration inhibitory factor/macrophages, and PD-L1/PD-1.1,5 A combination of signaling through surface receptors and gene mutations causes the constitutive activity of NF-κB and JAK-STAT pathways. In particular, HRS cells have constitutive activity of both canonical and alternative NF-κB pathways.10,53,54 In the canonical NF-kB signaling pathway, activation occurs through the stimulation of various receptors, including CD30, CD40, RANK, and OX40L. In the alternative NF-κB pathway, stimulation of the NF-κB-inducing kinase (MAP3K14) results in the activation of receptors such as CD40 and transmembrane activator and calcium-modulator and cyclophilin ligand interactor.10,53,54 The JAK-STAT pathway is activated by cytokines usually present within the cHL microenvironment. Activation of this pathway is also mediated by genomic gain or translocation of the JAK2 gene.55 A further constitutively active signaling pathway in HRS cells is the NOTCH1 pathway.56 NOTCH1 is highly expressed by HRS cells, and its ligand JAGGED1 is provided by cells in the microenvironment and by HRS cells themselves.56 In cHL cell lines, NOTCH1 signaling promotes HRS cell survival and proliferation.57,58
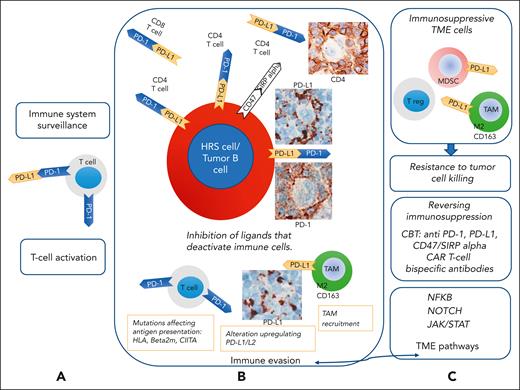
Cross talk between tumor cells and their cellular microenvironment. According to lessons learned from classic Hodgkin lymphoma (cHL), expression of PD-L1 can be found in tumor B cells, and expression of PD-1 is found on microenvironmental T cells in lymphomas with an inflammatory/immune cell-rich microenvironment. PD-1, which acts as an immunomodulatory molecule, is a negative regulator of activated T cells, B cells, and myeloid cells. PD-L1, a ligand of PD-1, is expressed on T cells, B cells, dendritic cells, and macrophages. (A) PD-L1 binds to PD-1 on T cells and regulates their activity. Binding of PD-1 to T cells with PD-L1 and 2 to HRS cells43 inactivates tumor-specific T cells, functioning as a checkpoint used by HRS cells and other tumor B cells to escape immune surveillance and evade immune destruction. (B) Classic HL with PD-L1 upregulation, acquired in HRS cells through highly recurrent copy gains of the chromosomal region containing the PD-L1 locus, is sensitive to PD-1 blockade therapy.12,13,44-46 In cHL and other lymphomas with inflammatory/immune cell-rich microenvironment, PD-L1 might be expressed by tumor cells and TAMs. PD-L1 binds to PD-1 on CD4+ and CD8+ T cells, attenuating the function of T cells and inhibiting the release of cytokines, leading to the immune escape of tumor cells. Following an antilymphoma immune response, lymphoma cells may recruit numerous TAMs and acquire genetic alterations that affect antigen presentation to T cells and upregulate the expression of PD-L1/L2. (C) Blockade of PD-1 and PD-L1 could be a therapeutic approach for enhancing immune cell function. Lymphoma cells might also be responsive to immunotherapy with antibodies that block CD47/SIRP (signal-regulatory protein) α interaction. Immune and inflammatory cell infiltrates in the TME produce molecules that bind to proteins expressed on the tumor cell membranes. The NF-κB, NOTCH1, and JAK-STAT pathways are activated via membrane-bound signaling. These TME pathways promote an inflammatory immune environment and resistance to apoptosis. Tumor infiltration by TAMs, immunosuppressive myeloid cells, and regulatory T cell may be related to the resistance to checkpoint blockade therapy (CBT). The mechanisms of resistance to PD-1 blockade have been identified in solid tumors partly,47,48 whereas they remain largely unknown in lymphoid malignancies. In solid tumors, a heavy infiltration of immune-suppressive myeloid cells can correlate with resistance to checkpoint blockade.49 Therefore, the characterization of the cHL microenvironment with a specific focus on inflammatory and immune cells might provide important information on the mechanisms of response to CBT.11,23 Furthermore, mutational analysis and clonal evolution measured by sequencing circulating cell-free tumor DNA may help identify the genetic determinants of resistance/refractoriness to anti–PD-1 therapy.50-52 Adapted and modified from Carbone and Gloghini.43
Cross talk between tumor cells and their cellular microenvironment. According to lessons learned from classic Hodgkin lymphoma (cHL), expression of PD-L1 can be found in tumor B cells, and expression of PD-1 is found on microenvironmental T cells in lymphomas with an inflammatory/immune cell-rich microenvironment. PD-1, which acts as an immunomodulatory molecule, is a negative regulator of activated T cells, B cells, and myeloid cells. PD-L1, a ligand of PD-1, is expressed on T cells, B cells, dendritic cells, and macrophages. (A) PD-L1 binds to PD-1 on T cells and regulates their activity. Binding of PD-1 to T cells with PD-L1 and 2 to HRS cells43 inactivates tumor-specific T cells, functioning as a checkpoint used by HRS cells and other tumor B cells to escape immune surveillance and evade immune destruction. (B) Classic HL with PD-L1 upregulation, acquired in HRS cells through highly recurrent copy gains of the chromosomal region containing the PD-L1 locus, is sensitive to PD-1 blockade therapy.12,13,44-46 In cHL and other lymphomas with inflammatory/immune cell-rich microenvironment, PD-L1 might be expressed by tumor cells and TAMs. PD-L1 binds to PD-1 on CD4+ and CD8+ T cells, attenuating the function of T cells and inhibiting the release of cytokines, leading to the immune escape of tumor cells. Following an antilymphoma immune response, lymphoma cells may recruit numerous TAMs and acquire genetic alterations that affect antigen presentation to T cells and upregulate the expression of PD-L1/L2. (C) Blockade of PD-1 and PD-L1 could be a therapeutic approach for enhancing immune cell function. Lymphoma cells might also be responsive to immunotherapy with antibodies that block CD47/SIRP (signal-regulatory protein) α interaction. Immune and inflammatory cell infiltrates in the TME produce molecules that bind to proteins expressed on the tumor cell membranes. The NF-κB, NOTCH1, and JAK-STAT pathways are activated via membrane-bound signaling. These TME pathways promote an inflammatory immune environment and resistance to apoptosis. Tumor infiltration by TAMs, immunosuppressive myeloid cells, and regulatory T cell may be related to the resistance to checkpoint blockade therapy (CBT). The mechanisms of resistance to PD-1 blockade have been identified in solid tumors partly,47,48 whereas they remain largely unknown in lymphoid malignancies. In solid tumors, a heavy infiltration of immune-suppressive myeloid cells can correlate with resistance to checkpoint blockade.49 Therefore, the characterization of the cHL microenvironment with a specific focus on inflammatory and immune cells might provide important information on the mechanisms of response to CBT.11,23 Furthermore, mutational analysis and clonal evolution measured by sequencing circulating cell-free tumor DNA may help identify the genetic determinants of resistance/refractoriness to anti–PD-1 therapy.50-52 Adapted and modified from Carbone and Gloghini.43
Evidence from gene expression profiling indicates that the composition of the TME varies depending on the Epstein Barr virus tumor status.59 Moreover, sequencing and gene expression studies in HRS cells suggest that the genotype and phenotype of HRS cells strongly influence cellular cross talk within the TME.23 Overall this cross talk, which shows the biological complexity of the disease, is essential for HRS cell growth and survival. Analogously, tumor cells of B-cell lymphomas other than cHL interact closely in association with the microenvironment cellularity to promote tumor progression and mediate immune evasion (Figure 2B-C).60
Mechanisms of action of immune checkpoint inhibitors
In several solid cancers, CD8+ cytotoxic T cells seem to be the main effector cells61 and recognize tumor cells through (neo)antigens presented in the context of major histocompatibility complex (MHC) class I. CD4+ T cells are more relevant than CD8+ T cells in checkpoint blockade therapy efficacy in cHL.62 Most cHL cases have MHC class I– HRS cells,62 and the inflammatory infiltrate in cHL is dominated by CD4+ T cells, which are often in direct contact with HRS cells and can recognize HRS cells via antigens presented in the context of MHC class II molecules. Interestingly PD-1+CD4+ T cells, are also enriched in the immediate proximity of PDL1+ HRS cells.63
In addition, the role of PD1+ NK cells and PD-L1+ monocytes/macrophages in the cHL TME has been hypothesized.64 The expansion of PD-1+ NK cells and PD-L1+ monocytes/macrophages is prominent in cHL. PD-1 blockade reverses immune evasion mediated by the interaction of PD-1+ NK cells and PD-L1+ monocytes/macrophages.64 Finally, a role of reverse signaling through PD-L1 into the HRS cells has also been suggested.65 Inhibition of reverse signaling through PD-L1 is an additional mechanism of action of checkpoint blockade therapy in cHL.
MHC class I and/or II downregulation may be a resistance mechanism to the PD-1/ PD-L1 blockade in cHL and other lymphomas. Several genes involved in antigen presentation are frequently mutated in cHL and primary mediastinal large B cell lymphoma (PMBCL). Deletion of 6p21.32, the locus for MHC class I and II genes, decreases surface expression of MHC class II and recurrently occurs in both cHL (43%)66 and PMBCL (32%),67 whereas it only rarely occurs (12%) in DLBCL.68 Structural variants of the class II MHC transactivator is a frequent gene fusion partner in lymphoma, and its alteration is found in 15% to 38% of PMBCL, 15% of cHL, and <5% of DLBCL.67,69,70 Therefore, a combination approach with PD-1 and CTLA-4 blockade or chimeric antigen receptor T-cell therapy may be required to bypass T-cell receptor and MHC interaction.71
Impact of anti–PD-1 therapy in lymphoma
Early-phase clinical trials have demonstrated the remarkable efficacy of anti–PD-1 in cHL12,72 and PMBCL,44 2 diseases that share the transcriptional profile and mechanisms of PD-L1 overexpression.73 Limited but promising data have also been reported for MGZL74 and THRLBCL.24 In contrast, the use of anti–PD-1 has been substantially disappointing in FL and DLBCL.21,25
Anti–PD-1 therapy has demonstrated a dramatic impact on the outcome of relapsed/refractory cHL, a clinical setting considered an unmet medical need before the availability of anti–PD-1 therapy.75,76 Initial trials using the anti–PD-1 antibodies nivolumab or pembrolizumab in patients for whom brentuximab vedotin and/or ASCT failed showed unprecedented response rates with average values of 22% complete remission and 65% partial remission, with a proportion of patients showing long-lasting duration of responses after either nivolumab12,45 or pembrolizumab.46,72 Based on these trials, the Food and Drug Administration granted accelerated approvals for nivolumab for the treatment of patients with cHL whose disease relapsed or progressed after ASCT and posttransplantation brentuximab vedotin and for pembrolizumab for the treatment of adult and pediatric patients with refractory cHL, or those who relapsed after 3 or more prior lines of therapy. More recent trials have investigated the efficacy of PD-1 blockade as a first-line therapy after allotransplant consolidation. Although the optimal setting in which to use these agents remains a matter of investigation, efficacy has been reported for all points during the disease course. Nonrandomized studies support the use of anti–PD-1 as consolidation therapy after ASCT for patients with cHL at high risk of relapse.77
Another interesting effect of exposure to anti–PD-1 therapy consisting of improved chemoresponsiveness observed among patients who were previously chemorefractory has been reported in retrospective studies of solid tumors,78 non-HL,79 and cHL.80-83 A good outcome has also been reported for ASCT after anti–PD-1 therapy in patients who are chemorefractory, challenging the dogma of chemosensitivity before ASCT. Merryman et al84 retrospectively reported 18-month progression-free survival of 75% among patients who had a positive pre-ASCT positron emission tomography–computed tomography.84 Moskowitz et al85 have recently reported the results of a trial incorporating anti–PD-1 therapy into second-line salvage therapy to improve the complete metabolic response rate before ASCT.85 Despite a formal experimental demonstration of the mechanism underlying the chemosensitizing effect of anti–PD-1 is lacking, one may hypothesize that changes of the TME may enable more effective chemotherapy activity by removing prosurvival signals from the TME.
Concluding remarks
For cHL, topological analysis revealed that the majority of PD-L1 in the TME is expressed by PD-L1+ TAMs, which physically colocalize with PD-L1+ HRS cells. PD-L1+ TAMs and PD-L1+ HRS cells are enriched in contact with CD4+ T cells, a subset of which are PD-1+. These data define a unique topology of cHL in which CD4+ T cells are a target of PD-1 blockade.63 Moreover, according to recent exploratory studies, the quantitative and spatial evaluation of immunomodulatory cells seems to be an important tool in the knowledge of the causes that favor the sensitivity or resistance to therapy.24 These data, although scientifically relevant, do not support the ability to use the microenvironment to identify lymphomas that will benefit from checkpoint inhibitors. They are not capable of precisely distinguishing whether molecules involved in signaling pathways are only close or in contact and do not reveal which cells and proteins influence the efficacy of immunotherapy in patients with individual lymphoma. Further research should explore innovative assays that can reveal which molecules predict sensitivity or resistance to therapy.
Acknowledgment
This work was supported in part by a grant from the Italian Association for Cancer Research (AIRC, grant #20575) (C.C.-S.).
Authorship
Contribution: A.C. designed the review; and A.C., A.G., and C.C.-S. contributed to writing and proofreading the manuscript.
Conflict-of-interest disclosure: C.C.-S. reports research support/funding from ADC Therapeutics, Sanofi, Roche; serves as adviser for Celgene/Bristol Myers Squibb, ADC Therapeutics, Karyopharm Therapeutics, Roche, and Sanofi; and receives honoraria from Bristol Myers Squibb, Merck Sharp & Dohme, Janssen Oncology, AstraZeneca, Incyte, Novartis, Takeda, and ADC Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Antonino Carbone, Centro di Riferimento Oncologico Aviano, Istituto Nazionale Tumori, IRCCS, Via F. Gallini 2, 33081 Aviano, Italy; e-mail: acarbone@cro.it.
References
Author notes
The project underlying this article, entitled, “Multiplexing immunohistochemistry of the tumor microenvironment (TME) in B-cell lymphomas,” was presented by A.C. at the Virtual Spring Meeting of the European Organization for Research and Treatment of Cancer (EORTC)–Pathobiology Group, 28 April 2022.
Data are available on request to the corresponding author, Antonino Carbone (acarbone@cro.it).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal