In this issue of Blood, Yoon et al1 show that patients with diffuse large B-cell lymphoma (DLBCL) at diagnosis exhibit a profound intestinal dysbiosis. The relative overrepresentation of Enterobacteriaceae family members is a robust and independent predictive factor of febrile neutropenia and relapse, despite first-line rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP) chemotherapy.
The RCHOP regimen has been considered the gold standard treatment for patients with DLBCL for >10 years, providing a progression-free survival (PFS) of >60% at 5 years.2 Despite numerous attempts to dethrone RCHOP by adding additional drugs (polatuzumab vedotin, lenalidomide, and ibrutinib), no drug combination has been able to outperform RCHOP so far.
Here, the authors showed that gut dysbiosis governs both toxicity and efficacy of RCHOP, paving the way to the development of microbiota-centered biomarkers and therapies.1
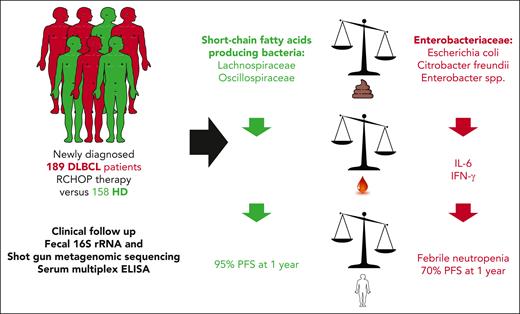
The authors analyzed the stool taxonomic composition of 189 patients newly diagnosed with DLBCL at baseline (excluding patients treated with antibiotics) using 16S rRNA and whole-genome shotgun sequencing, with 3 aims.1 First, they compared the metagenomic-based fecal repertoire from patients with DLBCL with that of 158 sex- and age-matched healthy donors (HDs), and correlated the gut microbiota compositional deviations with the incidence of febrile neutropenia and RCHOP failure (see figure). In this cohort, 86% of patients had a prolonged response, with a median follow-up of 16 months. Relapses usually occur within 24 months following RCHOP-based first-line treatment of DLBCL. Hence, this high response rate may be explained, at least in part, by the relatively short follow-up.
Study outline and results. Fecal 16S rRNA, shotgun metagenomic sequencing, and serum multiplex enzyme-linked immunosorbent assay (ELISA) were performed in a large cohort of newly diagnosed patients with DLBCL who were treated with RCHOP and in healthy individuals. Patients with DLBCL exhibit a profound dysbiosis, marked by high Enterobacteriaceae overrepresentation relative to short-chain fatty acid–producing bacteria. High relative abundance of Enterobacteriaceae in patients with DLBCL was associated with higher interleukin 6 (IL-6) and interferon gamma (IFN-γ) plasmatic concentrations, increased risk of febrile neutropenia, and lower PFS, compared with patients with no dominance of Enterobacteriaceae family members.
Study outline and results. Fecal 16S rRNA, shotgun metagenomic sequencing, and serum multiplex enzyme-linked immunosorbent assay (ELISA) were performed in a large cohort of newly diagnosed patients with DLBCL who were treated with RCHOP and in healthy individuals. Patients with DLBCL exhibit a profound dysbiosis, marked by high Enterobacteriaceae overrepresentation relative to short-chain fatty acid–producing bacteria. High relative abundance of Enterobacteriaceae in patients with DLBCL was associated with higher interleukin 6 (IL-6) and interferon gamma (IFN-γ) plasmatic concentrations, increased risk of febrile neutropenia, and lower PFS, compared with patients with no dominance of Enterobacteriaceae family members.
Nevertheless, patients with DLBCL showed reduced alpha and beta diversity of their gut microbiota composition compared with HDs. Most particularly, Enterobacteriaceae, including Escherichia coli, Citrobacter species (Citrobacter freundii, Citrobacter kasseri, and Citrobacter portucalensis), and Enterobacter species, were relatively more abundant in patients with DLBCL than in HDs. This was accompanied by a relative underrepresentation of health-related short-chain fatty acid (SCFA)–producing bacteria, including Prevotella copri, Lachnospiraceae (Fusicatenibacter saccharivorans, Anaerostipes hadrus, and Agathobacter rectalis), and Faecalibacterium species. The metagenomic signature was a robust predictor of hematological malignancy, allowing the authors to accurately discriminate HD from DLBCL-derived fecal samples (area under the curve, 0.89).
Of patients, 22% experienced febrile neutropenia, which led to a reduction in chemotherapy dose in 16 of 22 patients. Dose adaptation largely depends on medical attitude and clinical centers. Whether febrile neutropenia resulted in a delay in subsequent RCHOP was not clarified. Interestingly, patients experiencing febrile neutropenia had a relative enrichment in fecal Enterobacteriaceae (E coli and Klebsiella pneumoniae) and in tolerogenic species associated with resistance to immunotherapy (such as Enterocloster species [Enterocloster clostridioformis], Eggerthella lenta,3 and Clostridium innocuum4) with a parallel relative underrepresentation of SCFA producers compared with febrile neutropenia-free patients with DLBCL.
Moreover, a high relative abundance of Enterobacteriaceae family members was an independent prognosis factor strongly associated with shorter PFS, and outperformed the International Prognostic Index score (70% vs 95% 1-year PFS in patients with DLBCL with high vs low Enterobacteriaceae [dichotomized according to the 51st-100th vs 0-50th percentile]) (see figure). The detrimental effect of stage III/IV was erased in the presence of low Enterobacteriaceae abundance, meaning that stage I/II PFS Kaplan-Meier curve was superposable to that depicting PFS of stage III/IV DLBCL with low Enterobacteriaceae. Febrile neutropenia was not independently associated with a shorter PFS.
In parallel, the authors analyzed baseline cytokine plasma levels and found that high fecal Enterobacteriaceae relative abundance correlated with higher interleukin-6 (IL-6) and interferon gamma plasmatic concentrations. IL-6 is a proinflammatory cytokine induced by sepsis or endotoxemia and genotoxic stress, such as the one mediated by doxorubicin.5 It is tempting to speculate that IL-6 may interfere with chemotherapy efficacy and gut intestinal barrier fitness.
This study is the largest analysis of the prognostic impact of stool taxonomic composition in newly diagnosed patients with DLBCL ever described to date, highlighting the negative impact of the relative excess of intestinal Enterobacteriaceae for treatment toxicity and failure. The relative abundance of gut E coli/Shigella Enterobacteriaceae in patients with DLBCL compared with HDs was already reported in a smaller cohort of patients DLBCL,6 and may be in line with the cancer-associated stress ileopathy previously described.3 In autologous and allogeneic hematopoietic cell transplantation, the alpha diversity of pretransplant microbiota is lower than in HDs and further decreases during the course of transplantation.7 Loss of fecal diversity is associated with graft-versus-host disease and lower PFS and overall survival. In contrast, the presence of SCFA-producing bacteria correlated with improved response rates in patients with refractory B-cell acute lymphoblastic leukemia or B-cell lymphoma who were amenable to CD19 chimeric antigen receptor T-cell therapy.8 This study suggests a vicious circle between intestinal Enterobacteriaceae, systemic inflammation and immunosuppression, febrile neutropenia, and relapse. Anthracycline mediates immunogenic cell death and T-cell–dependent long-term clinical benefit. Therefore, restoring “gut eubiosis” and intestinal barrier fitness may represent a mandatory prerequisite for full-fledged RCHOP efficacy. Increasing the relative dominance of SCFA producers (using a high-fiber diet, specific prebiotics, probiotics,9 or complex microbial ecosystems10) should therefore be considered in front-line patients with DLBCL.
Conflict-of-interest disclosure: L.Z. is a founder of everImmune and the president of everImmune SAB and receives research funding from Daichi Sankyo, 9 Meters, and Pileje. C.B. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal