Abstract
Preventing viral infections at an early stage is a key strategy for successfully improving transplant outcomes. Preemptive therapy and prophylaxis with antiviral agents have been successfully used to prevent clinically significant viral infections in hematopoietic cell transplant recipients. Major progress has been made over the past decades in preventing viral infections through a better understanding of the biology and risk factors, as well as the introduction of novel antiviral agents and advances in immunotherapy. High-quality evidence exists for the effective prevention of herpes simplex virus, varicella-zoster virus, and cytomegalovirus infection and disease. Few data are available on the effective prevention of human herpesvirus 6, Epstein-Barr virus, adenovirus, and BK virus infections. To highlight the spectrum of clinical practice, here we review high-risk situations that we handle with a high degree of uniformity and cases that feature differences in approaches, reflecting distinct hematopoietic cell transplant practices, such as ex vivo T-cell depletion.
Introduction
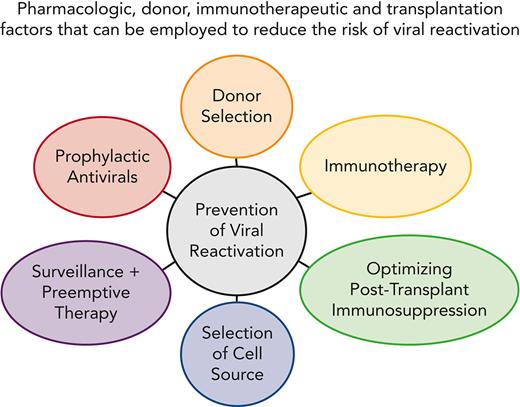
Although nonrelapse mortality (NRM) has decreased,1 infectious complications and latent viral infections in particular continue to significantly contribute to fatal outcomes, both alone and in the context of acute and chronic graft-versus-host disease (GVHD).2 Two major strategies have been used successfully to prevent viral infection and disease in hematopoietic cell transplant (HCT) recipients, preemptive therapy and prophylaxis. Advantages of a prophylactic strategy are the prevention of viral reactivation at all stages, simplicity, and high-efficacy, provided that the drug or immune intervention is nontoxic.3,4 Advantages of a preemptive approach are more targeted use of antiviral drugs and improved immune reconstitution because of antigen exposure.5-7 Progress in immune monitoring techniques have also permitted the development of hybrid prevention strategies that allow more accurate targeting of the at-risk population (Figure 1).3 In this article, we will discuss our approaches to several important viral reactivation scenarios of herpesviruses, adenoviruses (ADV), and BK virus (the prevention of hepatitis virus reactivations is reviewed elsewhere8-10).
CMV prevention strategies in HCT, including potential combined approaches. Red circles indicate weekly monitoring for CMV viremia; open circles indicate test time points that yielded viral loads below the threshold for the initiation of PET antiviral therapy; filled shapes indicate test time points with values above this threshold. Black arrows indicate the administration of antiviral PET. Black bars indicate administration of antivirals as prophylaxis. Blue triangles indicate administration of vaccine dose. All the strategies include clinical surveillance. aVaccination of transplant donor and/or recipient; bvarious vaccination schedules used; cvarious experimental vaccines currently being studied. D+R−, donor positive, recipient negative; Exp, experimental; IM, immune monitoring; PET, preemptive therapy; R+, recipient positive; RCT, randomizwd controlled trial; Tx, therapy. Reproduced with permission from Limaye et al.3
CMV prevention strategies in HCT, including potential combined approaches. Red circles indicate weekly monitoring for CMV viremia; open circles indicate test time points that yielded viral loads below the threshold for the initiation of PET antiviral therapy; filled shapes indicate test time points with values above this threshold. Black arrows indicate the administration of antiviral PET. Black bars indicate administration of antivirals as prophylaxis. Blue triangles indicate administration of vaccine dose. All the strategies include clinical surveillance. aVaccination of transplant donor and/or recipient; bvarious vaccination schedules used; cvarious experimental vaccines currently being studied. D+R−, donor positive, recipient negative; Exp, experimental; IM, immune monitoring; PET, preemptive therapy; R+, recipient positive; RCT, randomizwd controlled trial; Tx, therapy. Reproduced with permission from Limaye et al.3
Clinical impact of viral characteristics and transplantation techniques
The improvement and acceleration of immunological recovery is the “holy grail” for infection prevention after HCT. Multiple factors affect the duration of immunodeficiency and the associated risk and type of posttransplant infections, such as the conditioning regimen, donor type, graft source, and GVHD prophylaxis regimens and treatment.11-13 Balancing GVHD risk vs infection risk is challenging, because regimens that reduce GVHD risk (eg, anti-thymocyte globulin or ex vivo T-cell depletion) may have a trade-off of increased infectious complications.14-16 GVHD itself can also delay T-cell reconstitution by damaging thymic function.11,17 Recently, posttransplantation cyclophosphamide (PTCy) has been widely adopted to reduce chronic GVHD and studies on the infectious risk associated with PTCy regimens are emerging, with some showing a reduced risk of infection and disease compared with anti-thymocyte globulin (ATG),18-20 whereas others demonstrate similar or higher rates of infection (eg, cytomegalovirus [CMV], BK virus) than other GVHD prophylaxis regimens.21-25 T-cell depletion, in vivo26-29 or ex vivo,30 are associated with delayed immune reconstitution and viral infections. In contrast, sirolimus reduces the risk of CMV reactivation.31,32 The importance of donor immunity is well documented for CMV, and selection of donors based on serostatus is a well-established practice, as it reduces the risk of primary CMV infection or promotes CMV-specific immune reconstitution.33-35 The impact of donor immunity on recipient outcomes has also been demonstrated for herpes simplex virus (HSV), human herpesvirus 6 (HHV-6), and Epstein-Barr virus (EBV),36-39 but serostatus is not routinely used for donor selection for these viruses. HSV seropositive recipients with a seronegative donor (D−/R+) who received acyclovir prophylaxis for 30 days had an increased risk of both HSV wild-type and drug resistance disease in one study conducted.36 Donor HHV-6-specific CD4 T-cell immunity inversely correlated with early posttransplant HHV-6 viral load.38 EBV donor seropositivity modestly increased the risk of acute and chronic GVHD whereas recipient seropositivity increased the risk of acute and chronic GVHD, as well as nonrelapse and overall mortality.37
Pretransplant serologic assessment can result in false negative and false positive results due to hypogammaglobulinemia and passive transfer of antibodies, respectively. In clinical practice, it is important to be aware of this phenomenon and perform repeat testing in selected cases; however, at the authors’ institutions, we generally perform only one pretransplant test in donors and recipients. For CMV, where misclassification would be most consequential, weekly posttransplant virologic surveillance is performed, which would identify any misclassified patient and allow the start of preemptive therapy.
Understanding the immunological and viral mechanisms that affect viral reactivation, will facilitate the development of novel therapeutic interventions. The most significant progress that has been made in recent years is in the area of cellular immunotherapies,40 with late-stage trials now in progress.
Case 1
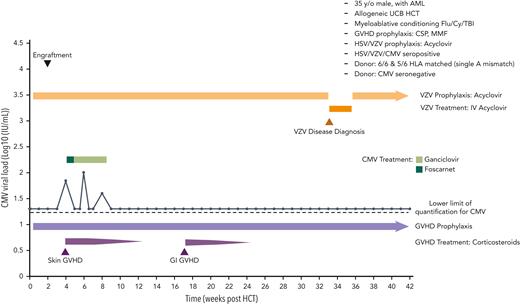
A 53-year-old, CMV seropositive woman with anaplastic large cell non-Hodgkin’s lymphoma was treated with alemtuzumab, followed by T-cell replete HCT from her matched-related CMV-seropositive brother (Figure 2). She received tacrolimus and mycophenolate mofetil (MMF) for GVHD prophylaxis. Antiviral prophylaxis included acyclovir 400 mg orally twice daily for HSV/VZV prophylaxis beginning on day −7 and letermovir 480 mg/day intravenously for CMV prophylaxis beginning on day 7. On day 14, the CMV viral load was 616 IU/mL. Induction of ganciclovir (5 mg/kg twice daily) was started and switched to foscarnet (90 mg/kg twice daily) 3 weeks later because of neutropenia and rising CMV DNAemia. After DNAemia clearance, we elected to use secondary prophylaxis with letermovir 480 mg orally daily for 14 weeks. Four weeks after stopping letermovir, the patient had recurrent CMV DNAemia, requiring induction therapy with valganciclovir 900 mg given orally every 12 hours, followed by foscarnet (90 mg/kg twice daily) due to neutropenia. After completion of treatment, no recurrences were observed off CMV antivirals by day 280.
Case 1: viral kinetics, antiviral prophylaxis, treatment, and relevant clinical data of a 55-year-old woman with anaplastic large cell lymphoma (ALCL) undergoing matched-related reduced intensity peripheral blood stem cell transplantation with CMV reactivation. HLA, human leukocyte antigens; PBC, peripheral blood cell; TBI, total body irradiation; VZV, varicella-zoster virus; y/o, years old.
Case 1: viral kinetics, antiviral prophylaxis, treatment, and relevant clinical data of a 55-year-old woman with anaplastic large cell lymphoma (ALCL) undergoing matched-related reduced intensity peripheral blood stem cell transplantation with CMV reactivation. HLA, human leukocyte antigens; PBC, peripheral blood cell; TBI, total body irradiation; VZV, varicella-zoster virus; y/o, years old.
At Memorial Sloan Kettering Cancer Center (MSK), we use letermovir prophylaxis for all CMV seropositive recipients.41 A comparison of the authors’ institutional guidelines on the use of letermovir and prevention strategy details for other viruses is shown in Table 1.
Selected viral reactivation prevention strategies at Fred Hutchinson Cancer Center, City of Hope National Medical Center and Memorial Sloan Kettering Cancer Center42
 |
 |
 |
 |
Strategies with a high degree of agreement are shaded gray. CTL, cytotoxic T lymphocyte; PCR, polymerase chain reaction; TCD, T-cell depletion; UCB, umbilical cord blood.
∗As of 11 May 2022.
†Continuation of prophylaxis at >1 year is based on risk for VZV reactivation.
‡CD4 T cells >200 cells/μL; CD19 B cells >50 cells/μL; immunoglobulin G >400 mg/dL; >8 weeks after last dose of IV immunoglobulin; minimum PHA of 40%; >6 months after last dose of rituximab.
§Determined by the transplant physician and dependent on time posttransplant, donor type, and availability of donor leukocytes or donor EBV specific CTLs and suspicion of PTLD.
‖High risk setting: ADV viral load: <day 30, >200 copies/mL; day 30 to 100, >1000 copies/mL; >day 100, >10 000 copies/mL.
At MSK, we began letermovir on day 7 for higher-risk HCT recipients, as defined in the phase 3 clinical trial,45 and by day 28 for lower risk patients, based on differences in time to CMV reactivation. T-cell replete and matched-related donor HCT are typically considered low risk; however, prior treatment with alemtuzumab27 put our patient at higher risk; thus, letermovir was started on day 7. We routinely check CMV PCR before starting letermovir; however, in this case, it was not checked. The most plausible explanation for breakthrough CMV infection here is active CMV replication at the time of letermovir initiation.46 Suboptimal exposure could be considered if letermovir was administered orally in the setting of severe mucositis or GVHD with vomiting or diarrhea.47 Letermovir resistance would be unlikely after 7 days of prophylaxis but is reported to develop when letermovir is used for treatment.48,49 The DNAemia threshold for initiating preemptive therapy for patients on letermovir is based on patient risk and type of assay used for monitoring. Delay in starting preemptive therapy in high-risk patients has been associated with prolonged DNAemia.50,51
During both CMV episodes, our patient had rising DNAemia after 2 weeks of ganciclovir induction therapy, prompting CMV resistance testing,52 which did not reveal any resistance mutations. Foscarnet was initiated to offset the ganciclovir-induced neutropenia, as maribavir was not available at the time. Maribavir is currently approved by the US Food and Drug Administration for the treatment of refractory and resistant CMV.53,54 Maribavir is orally bioavailable and is not associated with myelosuppression or nephrotoxicity, and for the most part, maintains activity against CMV strains resistant to other antivirals (see commonly identified resistance mutations in Figure 3). Disadvantages include the potential development of resistance in the setting of persistent immunosuppression and ineffectiveness in treating retinitis or central nervous system infections.55,56 Because our patient had severe T-cell lymphopenia (CD4+ T-cell count, 30 cells/μL), we elected secondary prophylaxis with letermovir 480 mg orally daily for 14 weeks (www.clinicaltrials.gov, #NCT04017962). Low level DNAemia (<137 IU/mL) while on letermovir, was monitored off treatment because viral blips likely represent long DNA concatemers rather than infectious virions.57,58 To date, our data support letermovir as secondary prophylaxis in agreement with the French compassionate-use experience.59 Ganciclovir or its oral prodrug, valganciclovir, may be used for secondary prophylaxis but are associated with dose-dependent neutropenia.60 Valganciclovir achieves similar exposure as intravenous ganciclovir in patients with stable GVHD61 and has shown similar efficacy for treatment of CMV infection.62
Venn diagram showing the most common mutations conferring resistance to CMV antivirals. Drugs for treatment of CMV are labeled in each oval, with the abbreviations listed in parentheses. The proteins (bold and underlined) and specific amino acid mutations commonly found to confer drug resistance are shown below the drug names. Mutations associated with resistance to more than 1 drug are shown in the overlap between multiple ovals. Figure adapted from Chou.63
Venn diagram showing the most common mutations conferring resistance to CMV antivirals. Drugs for treatment of CMV are labeled in each oval, with the abbreviations listed in parentheses. The proteins (bold and underlined) and specific amino acid mutations commonly found to confer drug resistance are shown below the drug names. Mutations associated with resistance to more than 1 drug are shown in the overlap between multiple ovals. Figure adapted from Chou.63
Considerable pharmacokinetic interpatient variability has been observed for ganciclovir but no association between therapeutic drug monitoring and clinical outcomes has been identified.64 Therapeutic monitoring is not readily available in the United States and not recommended for adult patients.52 Limited experience with ganciclovir therapeutic monitoring comes from European centers4 and selected pediatric patients.
Rebound CMV DNAemia after discontinuation of letermovir prophylaxis is common after haploidentical, cord, and T-cell depleted HCT or on corticosteroids,65-68 and may require shorter treatment than DNAemia early post-HCT.65,69 At MSK, we continued letermovir prophylaxis beyond day 100 (until immune reconstitution) in high-risk patients with sustained viral suppression through day 180.70-73 In a randomized trial, extended duration letermovir prophylaxis for 200 days was superior to 100 days in preventing clinically significant CMV infection (www.clinicaltrials.gov, #NCT03930615). We continue weekly CMV monitoring for at least 4 to 6 weeks after stopping letermovir. A risk-adapted strategy for late letermovir was used in the City of Hope (Table 1). Diminished cell-mediated responses in vitro have been observed in patients receiving letermovir prophylaxis compared with those treated preemptively.7,74,75 A robust correlation between in vitro findings and clinical outcomes in the era of letermovir is needed before incorporating routine immune monitoring into clinical practice. Immunological monitoring, in addition to virological monitoring, may aid in personalizing CMV management after HCT (Figure 1).
Case 2
A 35-year-old man with high-risk acute myelogenous leukemia in complete remission (CR) underwent umbilical cord blood (UBC) HCT following myeloablative conditioning with fludarabine, cyclophosphamide, and TBI (Figure 4). The patient was HSV type 1 (HSV-1), VZV, and CMV seropositive (donor CMV seronegative). He received high-dose acyclovir (800 mg twice daily) for the prevention of HSV and VZV and PCR surveillance for CMV reactivation. Neutrophil engraftment occurred on day 13, and grade 2 acute GVHD (skin and upper gastrointestinal [GI] tract) was diagnosed on day 32 and treated with systemic corticosteroids. Weekly surveillance for CMV with PCR was positive on day 28 (viral load, 92 IU/mL) and was initially treated with foscarnet (due to neutropenia) and subsequently with ganciclovir. On day 234 after HCT, the patient developed a disseminated vesicular rash, which was confirmed to be VZV by PCR. There were no signs of hepatic, GI, pulmonary, or central nervous system involvement. The patient was started on 10 mg/kg every 8 hours of intravenous acyclovir and recovered.
Case 2: antiviral prophylaxis, treatment, and other relevant clinical data of a 33-year-old man with AML undergoing cord blood transplantation for disseminated VZV infection. AML, acute myeloid leukemia; CSP, cerebrospinal; Cy, cyclophosphamide; Flu, fludarabine.
Case 2: antiviral prophylaxis, treatment, and other relevant clinical data of a 33-year-old man with AML undergoing cord blood transplantation for disseminated VZV infection. AML, acute myeloid leukemia; CSP, cerebrospinal; Cy, cyclophosphamide; Flu, fludarabine.
There is high-quality evidence that antiviral prophylaxis with acyclovir, valacyclovir, or similar agents is highly effective in preventing VZV in HCT recipients.76,77 Society guidelines recommend at least 1 year of antiviral prophylaxis.34 However, VZV disease after discontinuation of prophylaxis is common in highly immunosuppressed patients.34 Therefore, prolonged prophylaxis is recommended, although the duration is not well defined. Unfortunately, biomarkers such as CD4 T-cell counts are not reliable.78,79 At Fred Hutch, we continue prophylaxis until 6 months after discontinuation of systemic immunosuppression. In UBC transplant recipients, prophylaxis is routinely continued for at least 3 years after transplantation, regardless of the presence of systemic immunosuppression due to the inherent delayed immune reconstitution associated with UCB blood transplantation,79 a practice that was not in place at the time this case occurred. A wide range of dosing regimens have been used for antiviral prophylaxis. At the Fred Hutchinson Cancer Center (Fred Hutch), we used a relatively high-dose regimen (acyclovir, 800 mg twice daily; valacyclovir, 500 mg twice daily) based on an earlier randomized trial.76 This dose is higher than the often used 400 mg twice a day dose, but still significantly lower than doses proposed to prevent CMV in earlier trials (3200 mg/day of acyclovir or 8 g/day of valacyclovir). A dose of 800 mg twice a day prevented the emergence of interval drug resistance to HSV among patients who are HSV seropositive.80 If the goal is to primarily target VZV, eg, in VZV seropositive and HSV seronegative cases, lower doses of antiviral agents can effectively prevent VZV reactivation.34 It is also important to note that visceral VZV disease without concomitant skin rash presentation is possible and constitutes a life-threatening condition. Physicians should be vigilant and perform plasma PCR testing and start empiric treatment in patients presenting with severe abdominal pain and rapidly rising transaminases, especially when the patient is not receiving antiviral prophylaxis.81,82 The strategies used at MSK and the City of Hope National Medical Center (COH) are shown in Table 1.
Recently, vaccination against VZV has been shown to reduce VZV reactivation disease in autologous transplant recipients,83 and an adjuvanted subunit vaccine has been approved for autologous HCT recipients.84 There are limited data on how to effectively use that vaccine in high-risk allograft recipients.85-87 At this time, antiviral prophylaxis remains the primary strategy in high-risk allograft recipients; at Fred Hutch, we recommend no adjuvanted vaccines until 24 months after HCT and ≥6 to 8 months off immunosuppressive therapy and no flare of GVHD in allograft recipients. The guidelines for the MSK and COH are presented in Table 1.
Case 3
A 31-year-old man underwent double UCB HCT for treatment of acute myeloid leukemia after conditioning with fludarabine, cyclophosphamide, and TBI (13.2 Gy). He was CMV seronegative, HSV, and VZV positive. He received posaconazole, acyclovir, and dapsone prophylaxis, followed by weekly surveillance for CMV using PCR. On day 16, with engraftment, he developed a rash consistent with engraftment syndrome, which was treated with a 3-day course of systemic corticosteroid (1 mg/kg). The patient did not show any evidence of residual disease or GVHD. An episode of Streptococcus mitis bacteremia was treated with vancomycin. A computed tomography scan on day 60, performed for follow-up of a pretransplant residual lung infiltrate following SARS-CoV-2 pneumonia, showed scattered bilateral pulmonary nodules compatible with multifocal pneumonia, possibly due to fungal etiology. A bronchoscopy with bronchoalveolar lavage (BAL) revealed low colony counts of different bacteria and was negative for CMV, respiratory viruses, fungal pathogens, and a broad range of bacteria. Because posaconazole levels were low, a switch to isavuconazole was recommended, and testing of the BAL fluid for HHV-6 DNA was requested, which returned to 280 copies/mL. Although the clinical symptoms continued to be mild, valganciclovir (900 mg/kg twice daily) was added to the empiric antifungal regimen for 4 weeks. The patient’s condition slowly improved with resolution of pulmonary infiltrates.
Recent studies have provided high-quality evidence that HHV-6 is a pulmonary pathogen in HCT recipients and that viral load in the BAL may be used to decide whether treatment is warranted.88-90 Similar to CMV, another beta herpesvirus, HHV-6 DNA can be detected at low levels in the BAL in asymptomatic patients, a phenomenon known as pulmonary shedding.89,91 But contrary to CMV, strong evidence has been lacking until recently that HHV-6 can also cause lung disease. Three studies added significant strength to the argument that HHV-6 is a pulmonary pathogen.88-90 The most recent work by Hill et al. demonstrated that a viral load of >250 copies/mL is associated with increased mortality in patients with and without a pulmonary co-pathogen,88 and that this threshold is also associated with HHV-6 mRNA detection.92 HHV-6 can be integrated into the genome and passed through the germ line, which will result in a positive detection.
In our patient, BAL did not show evidence of fungal pathogens by antigen and molecular methods; however, the radiographic and clinical presentations were consistent with invasive fungal disease. Noninfectious etiologies, such as cryptogenic organizing pneumonia, were also considered. Although the clinical presentation was not classic for viral pneumonia, given the high-risk setting for HHV-6 complications93 and recent data that support HHV-6 as a pulmonary pathogen, we opted to add antiviral treatment with valganciclovir to the empiric treatment including coverage against invasive mold infections, which led to a slow resolution of pulmonary infiltrates.
Presently, we do not recommend routine surveillance for HHV6 by PCR, prophylaxis or preemptive treatment of viremia, and testing should be based on a syndromic approach (eg, CNS symptoms, unexplained pneumonitis, etc).43 Prophylaxis and preemptive therapy in prospective, nonrandomized therapy were found to be ineffective in preventing CNS infection (encephalitis),94-98 and to date, no randomized clinical trials have demonstrated the benefit of these approaches with currently available drugs or immunotherapies. However, experimental approaches are being studied.40,99 Chromosomally integrated HHV-6 occurs in ∼1% of recipients and donors and has been associated with an increased risk of acute GVHD and CMV viremia.100-102 It can confound PCR detection of HHV-6 in blood, cerebrospinal fluid, and BAL fluid, but can be ruled out diagnostically by testing for HHV-6 PCR DNA in a hair follicle, nail clipping, or by digital droplet PCR.101,102 Approaches to manage HHV-6 at the authors’ institutions are shown in Table 1.
Case 4
A 25-year-old man with acute lymphoblastic leukemia in CR received a double UCB HCT following reduced intensity conditioning with cyclophosphamide, thiotepa, fludarabine, and TBI (400 cGy).103 The GVHD prophylaxis included cyclosporine and MMF. Antiviral prophylaxis included acyclovir for HSV/VZV, and letermovir (240 mg/day because of cyclosporine) for CMV.
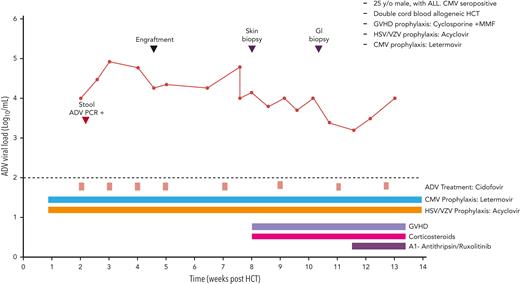
On day 14, ADV viral load in the plasma was 11 000 copies/mL on routine monitoring. ADV was also detected in stool samples using qualitative PCR. Cidofovir (CDV) 5 mg/kg once weekly was started, along with probenecid and hydration. Viral load trends and clinical course are summarized in Figure 5. Neutrophil engraftment occurred on day 32. The hospital course was complicated by severe acute GVHD of the skin and gut, which required corticosteroids and additional immunosuppressants. Lower endoscopy with biopsy was consistent with GVHD without evidence of ADV colitis. The patient received 9 doses of cidofovir, the last dose in week 16. The kidney function remained within normal limits. Subsequent ADV viral loads remained <1,000 copies/mL by week 24. His GVHD was controlled with low dose steroids and ruxolitinib.
Case 4: viral kinetics, antiviral treatment, and relevant clinical data of a 25-year-old man with ALL undergoing double cord blood transplantation with adenovirus infection. ALL, acute lymphocytic leukemia.
Case 4: viral kinetics, antiviral treatment, and relevant clinical data of a 25-year-old man with ALL undergoing double cord blood transplantation with adenovirus infection. ALL, acute lymphocytic leukemia.
The main risk factors for ADV viremia post-HCT are donor mismatch (cord blood, haploidentical, or HLA mismatched parent), T-cell depletion (ex vivo CD34 selection or alemtuzumab), grade III-IV GVHD, and severe lymphopenia (<0.2 × 109/L).104,105 ADV viremia occurs more frequently in pediatric than adult HCT (14%-16% vs 3%, respectively, in survey studies); the true incidence may be underestimated, particularly in adults, because of lack of routine monitoring. High-levels of ADV viremia >1,000 copies/mL occur in 9% of pediatric and 2% of adult HCT and correlate with increased mortality.106,107 Among ex vivo T-cell depleted HCT at MSK, 7% and 17% of adult and pediatric HCT, respectively, developed ADV viremia by day 100. Clinical outcomes were similar in both groups. The magnitude and duration of ADV viremia (expressed as adjusted area under the curve of viremia by Day 100 post-HCT) strongly correlated with mortality.108,109
Because ADV infection is more common in children, most of the published literature comes from pediatric centers. Monitoring high-risk patients and early intervention at viral load thresholds ≥102–3 copies/mL in the blood are associated with improved survival.110 In children, the GI tract is a reservoir for the persistence and replication of ADV. A concentration of ADV ≥106 copies/g of feces is predictive of ADV infection and disseminated disease and may precede ADV viremia by median of 11 days.104,111,112 Unlike children, the role of the gastrointestinal tract as a reservoir for persistence, replication, and expansion of ADV in adult immunocompromised patients is not certain, thus monitoring ADV in stool is not recommended for adults.113
At MSK, we routinely monitor plasma ADV in mismatched, UCB, haploidentical, and T-cell depleted HCT. We initiate preemptive therapy at 102-103 copies/mL during the first 30 days post-HCT (Table 1). For symptomatic patients, we recommend tissue sampling and staining for ADV, as the clinical presentation of ADV disease may be indistinguishable and co-exist with GVHD or other infections. Currently, there is no approved therapy for ADV. CDV is used off label,105 with nephrotoxicity and myelosuppression being the major dose-limiting toxicities.114,115 Brincidofovir (BCV), an experimental lipid conjugate of CDV that delivers higher concentrations of the active moiety (CDV diphosphate) in cells, demonstrated robust reduction of ADV viremia in clinical trials;116-118 however, further development was halted because of GI toxicity. An intravenous formulation of BCV is presently being studied (www.clinicaltrials.gov, #NCT04706923). Immune reconstitution is key for controlling ADV.28,119 Enhancing ADV-specific T-cell immunity by adoptive transfer of donor-derived or third party-derived ADV-specific CTL as a bridge to immune recovery has been used with success in phase 2 studies.40,120 Currently, ongoing randomized trials of third party multivirus-specific T cells for prevention (www.clinicaltrials.gov, #NCT05305040) and treatment of ADV (www.clinicaltrials.gov, #NCT05179057) will inform the potential of this promising form of therapy. ADV-CTL were considered for our patient but not pursued because of active GVHD and corticosteroid doses >0.5 mg/kg.
Case 5
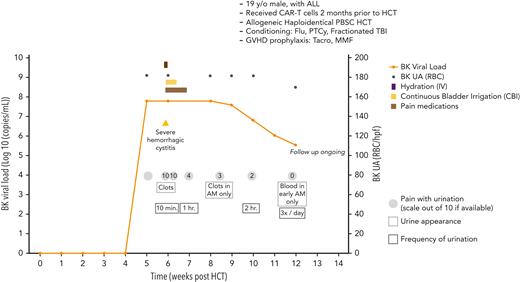
A 19-year-old male with relapsed/refractory acute lymphoblastic leukemia (initial induction chemotherapy with modified hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) regimen and rituximab, achieved CR after the first cycle and at cycle 4 had relapsed disease and subsequently multiple lines of therapy that failed to induce remission). He then underwent chimeric antigen receptor T (CAR-T) cell therapy (tisagenlecleucel [Kymriah]), which was complicated by cytokine release syndrome and macrophage activation syndrome treated with dexamethasone, tocilizumab, anakinra, and emapalumab (Figure 6). Subsequently, he was noted to have MRD+ on a bone marrow biopsy. Two months after receiving CAR-T cells, he underwent haploidentical peripheral blood (PB) HCT after conditioning with fludarabine, fractionated TBI, and PTCy (2 doses) and immunosuppression with tacrolimus and MMF. On day 35, he presented with dysuria and the BK virus load in urine was > 62 million copies/mL (no BK viremia in plasma), and 7 days later started frankly bloody urine and clots in urine associated with frequent urination with severe pain (10/10) for which he was hospitalized. He was managed conservatively with aggressive intravenous hydration and continuous bladder irrigation for 72 hours (the Foley catheter was then removed), and tamsulosin was subsequently added. Cidofovir and other antibacterial quinolones were not used. (He remained symptomatic for many weeks, and his course of illness is depicted in Figure 6.)
Case 5: viral load, clinical symptoms, and other relevant clinical data of a 19-year-old man with ALL undergoing PBSC transplant and BK virus associated hemorrhagic cystitis. ALL, acute lymphocytic leukemia; CBI, continuous bladder irrigation; IV, intravenous; PBSC, peripheral blood stem cell; RBC, red blood cells; Tacro, tacrolimus; UA, urine analysis.
Case 5: viral load, clinical symptoms, and other relevant clinical data of a 19-year-old man with ALL undergoing PBSC transplant and BK virus associated hemorrhagic cystitis. ALL, acute lymphocytic leukemia; CBI, continuous bladder irrigation; IV, intravenous; PBSC, peripheral blood stem cell; RBC, red blood cells; Tacro, tacrolimus; UA, urine analysis.
The risk factors for the development of BK virus HC are age >7 years, unrelated and haploidentical donors, cord and peripheral blood stem cells, myeloablative conditioning, GVHD grades II-IV, and BK virus viral load >107 and >103 in urine and blood, respectively.44,121 There is no proven or recommended therapeutic intervention for the prevention of BK virus related hemorrhagic cystitis.44 Monitoring for BK virus reactivation in urine or plasma is not recommended for routine clinical care. HCT patients with symptoms or signs of cystitis should be tested for polyomaviruses (and ADV) to avoid unnecessary use of antibacterial agents, especially those with negative urine cultures. The mainstay of hemorrhagic cystitis management is symptomatic relief of pain, continuous bladder irrigation in situations of extensive clots, pain management, and reduction of immunosuppression if possible. We do not recommend systemic treatment with cidofovir because of the lack of proven efficacy and the risk of nephrotoxicity.122 In special situations, intravesical cidofovir may be used with caution because of the lack of clinical trial data. Those with severe disease may require urological interventions. New developments include immunotherapy with off-the-shelf multivirus-specific T cells,123 currently in phase 3 randomized clinical trials, both for prevention and for treatment.
Summary and conclusions
High-quality evidence information exists for the effective prevention of HSV, VZV, and CMV. However, despite these advances, significant knowledge gaps remain. Given the strong associations between HHV-6 and ADV viral loads and various poor clinical outcomes, randomized controlled trials should be conducted to evaluate both preemptive and prophylactic antiviral and cellular therapy strategies. BK virus related hemorrhagic cystitis is a debilitating infection that often leads to hospitalization in severe cases in patients undergoing high-risk HCT, and the only options are the institution of symptom control measures. In addition, a systematic evaluation of immune monitoring strategies as adjuncts to preemptive and prophylactic strategies is needed (Figure 1). After decades of development, virus-specific cellular therapies, both single- and multi-target, as well as new vaccines, most notably for CMV prevention, are being tested in clinical trials. Likewise, an impressive body of literature has increased our knowledge of viral immunopathogenesis of DNA viruses in high-risk transplant settings, which can be used to some extent to modulate reactivation risk. Our rapidly increasing knowledge of how the microbiome affects transplant outcomes through its impact on GVHD risk and severity124,125 or by affecting viral host defense mechanisms,126 as well as novel immunotherapies,40 may open a path to innovative prevention strategies in the future.
Acknowledgments
The authors thank Joshua Hill, Pooja Bhattacharyya, and Jim Boonyaratanakornkit for assistance with case identifications and descriptions; and Rachel Blazevik for creating figures. They also thank Ashley Sherrid and Jessica L. Lawler for editorial assistance.
This work was supported in part by National Institutes of Health National Cancer Institute (NCI) core support grant CA033572 (S.S.D.), National Institutes of Health NCI core support grant P30 CA008748 (G.A.P.), and the National Institute of Health Institute of Allergy and Infectious Diseases (HHSN272201600015C) (M.B.).
Authorship
Contribution: All authors submitted case presentations and discussions and were involved in the writing of the manuscript.
Conflict-of-interest disclosure: S.S.D. has received research support (funds paid to institution) from Merck, Ansun Biopharma, AlloVir, Gilead, Geovax, Amplyx, and Karius; consulting/advisory board fees from Merck, AlloVir, and Aseptiscope; and speakers’ bureau fees from Takeda, Merck, and Astellas, all outside the submitted work. G.A.P. has received research support (funds paid to institution) from Merck and Shire Pharmaceutical (now known as Takeda) and consulting/other fees from AlloVir, Amplyx, Cidara, Merck & Co, Octapharma, SLC Behring, Takeda, SymBio, and Vera, all outside the submitted work. M.B. has received research support (funds paid to institution) from Astellas, Gilead Sciences, Shire Pharmaceutical (now known as Takeda), and Merck & Co Inc; consulting fees from Merck & Co Inc, AlloVir, and SymBio; and consulting fees and the rights to acquire equity from EvrysBio and Helocyte, all outside of the submitted work.
Correspondence: Michael Boeckh, Fred Hutchinson Cancer Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: mboeckh@fredhutch.org.
References
Author notes
∗S.S.D. and G.A.P. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal