In this issue of Blood, Harker-Murray et al present data from the Checkmate744 phase 2 study, using 2 novel-novel combinations, in an international response-adapted approach for children, adolescents, and young adults (CAYA) with relapsed/refractory (R/R) classic Hodgkin lymphoma (cHL).1 Building on excellent outcomes of both brentuximab vedotin (BV) plus nivolumab (NIVO)2 and BV plus bendamustine (BENDA) in the R/R setting,3,4 a response-adapted approach was used to determine the complete metabolic response (CMR) rate at the end of induction with BV-NIVO or intensification with BV-BENDA, and at any point before consolidation with autologous stem cell transplant (ASCT).
Although the prognosis for patients with cHL in the CAYA population is outstanding, those with R/R cHL treated with ASCT have a 5-year relapse rate of 41% and a 10-year progression-free survival (PFS) rate of only 47%. Failure to achieve CMR before ASCT is one of the most important predictors of relapse; therefore, it is critical to utilize regimens that induce deep responses quickly.5 Furthermore, for those who remain in remission, over half the deaths have been attributed to nonrelapse mortality, underscoring the need for salvage therapies that balance the long-term toxicities, like secondary malignancies, cardiovascular and pulmonary toxicity, and gonadal dysfunction, in the CAYA population. Hence, the authors’ goal is to identify a response-adapted salvage strategy with high CMR rates while minimizing long-term toxicity.
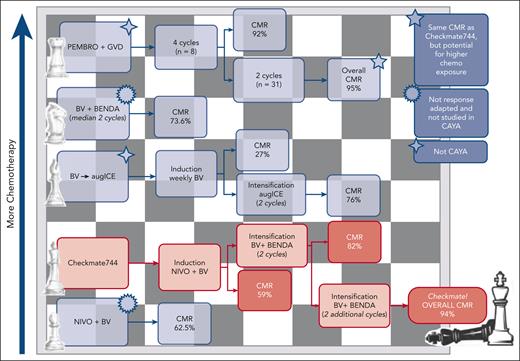
The authors present data from the standard-risk CAYA cohort, enrolling 44 patients with a median age of 16 years. Patients were treated with BV-NIVO induction for up to 4 cycles, achieving a CMR rate of 59%. Eleven patients did not experience CMR, and they proceeded to intensification with BV-BENDA, increasing the overall CMR rate to 94%. Altogether, using this approach, the 1-year PFS rate was excellent at 91%. It is meaningful when interpreting the results that the use of maintenance BV or radiotherapy in the post-ASCT period was not uniform, potentially impacting the overall PFS.
One aim of Checkmate744 was identifying an effective salvage regimen that minimized toxicity. The data successfully demonstrated few hematologic toxicities and adverse events leading to discontinuation; this is a stark contrast when compared with the adverse effect profiles of conventional salvage chemotherapy. As expected, grade 3/4 treatment-related adverse events were higher in the intensification phase vs the induction phase (27% [3/11] and 18% [8/44], respectively), the most common of which were vomiting and cytopenia. Notably, within the induction phase, there was only 1 observed grade 3/4 immune-mediated infusion-related reaction. There were no treatment-related deaths. However, with a short follow-up of only 20.9 months, it is difficult to ascertain the long-term toxicity profile of this regimen. It is reasonable to assume that this response-adapted strategy is less likely to result in secondary malignancy and end-organ damage compared with conventional combination salvage chemotherapy.
An important consideration for patients with R/R cHL is identifying a salvage regimen that does not preclude effective stem cell mobilization or complications peri-ASCT. BENDA is associated with prolonged and sometimes irreversible cytopenias, yet within this cohort, a median of only 1 apheresis session allowed for effective collection of CD34+ stem cells. In addition, there have been reports of engraftment syndrome in patients receiving checkpoint inhibitors before ASCT; however, the authors of Checkmate744 did not observe this phenomenon. Hence, this approach did not appreciably hinder successful ASCT.
Several questions remain: What is the right opening move in R/R cHL? How does this salvage therapy, employing a checkpoint inhibitor and antibody-drug conjugate, compare with others with a similar backbone? Two prior independent studies employing BV-NIVO illustrated CMR rates of 61% to 62.5%.2,6 These findings are similar to the 59% CMR rate reported in the induction cohort in Checkmate744. Other trials employing BV-BENDA in the R/R setting observed a CMR of 43% to 73.6% in various lines of treatment.2,4 How these trials compare with the dose intensification cohort of Checkmate744 is difficult to discern, although they do provide a rationale for the best next move in the R/R CAYA population.
How does the Checkmate744 regimen compare with other response-adapted checkpoint inhibitor or BV-chemotherapy combinations? A phase 2 study evaluating pembrolizumab plus gemcitabine, vinorelbine, and liposomal doxorubicin demonstrated an impressive 95% CMR rate7 that is similar to the overall CMR rate in Checkmate744; however, it does not address avoidance of chemotherapy in the CAYA population and does not abrogate the concern of long-term toxicity. Another response-adapted approach was the BV-augmented ifosfamide, carboplatin, and etoposide trial, exposing >70% of patients to higher than standard doses of chemotherapy, achieving an overall 76% CMR. This approach, although effective, further increases potential long-term toxicity.8 These are 2 examples of the many salvage therapies combining BV or checkpoint inhibitors with chemotherapy (see figure).
Selected salvage therapies combining BV or checkpoint inhibitors with chemotherapy are depicted. Regimens are listed with increasing use of chemotherapy from bottom to top. augICE, augmented ifosfamide, carboplatin, etoposide; GVD, gemcitabine, vinorelbine, pegylated liposomal doxorubicin; PEMBRO, pembrolizumab.
Selected salvage therapies combining BV or checkpoint inhibitors with chemotherapy are depicted. Regimens are listed with increasing use of chemotherapy from bottom to top. augICE, augmented ifosfamide, carboplatin, etoposide; GVD, gemcitabine, vinorelbine, pegylated liposomal doxorubicin; PEMBRO, pembrolizumab.
Finally, important to consider in the calculus of determining the best salvage regimen is the consideration of BV exposure in the frontline setting. Unfortunately, none of the 44 patients enrolled in this study was previously exposed to BV. Data from the ECHELON-1 trial have demonstrated an overall survival benefit in patients with newly diagnosed high-risk cHL.9 Recently, Castellino et al presented the effect of frontline BV in a large multicenter pediatric cohort, demonstrating a 59% reduced risk of death.10 These data support extending frontline BV-based therapy to the high-risk CAYA cHL population. The hope is with this shift in treatment strategy, fewer patients will relapse, requiring salvage therapy. However, for those who do unfortunately relapse, how these results will impact BV-based salvage treatment is yet to be defined. Retreatment with BV has been demonstrated to be effective in some, and synergy between BV and checkpoint blockade may potentially overcome previous exposure.
The importance of the Checkmate744 study is multifaceted. Herein, the authors have established a response-adapted approach to achieve high CMR rates while limiting exposure to chemotherapy in the CAYA population. They have achieved this through international cooperation to harmonize risk evaluation and treatment strategies. In these exciting times where treatment options for cHL are rapidly expanding, Checkmate744 brings us one step closer to choosing the right sequence of moves for capturing the win.
Conflict-of-interest disclosure: J.E.A. is on the Advisory Board for AstraZeneca. S.S.T. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal