In this issue of Blood, Li et al1 demonstrate in vivo gene correction of sickle cell disease in mouse hematopoietic stem cells (HSCs) through intravenous delivery of a prime editor–expressing vector.1
Clinical application of gene therapy for sickle cell disease and other monogenic hematopoietic diseases remains challenging, due to cytotoxic and mutagenic risks associated with delivery of gene therapy vectors into patient HSCs ex vivo and issues with cytoreductive conditioning regimens for stem cell transplant. Recently, insertional oncogenesis occurred in several patients with cerebral adrenoleukodystrophy, following ex vivo gene therapy, and was attributed to the strong internal promoter used to drive gene expression in the vector,2 highlighting one potential consequence of dysregulated gene expression driven by a foreign promoter. The inclusion of chromatin insulator elements within lentiviral vectors to protect against dysregulating promoter effects resulted in an unexpected clonal perturbation of hematopoietic reconstitution following transplant in separate clinical trials for β-thalassemia3 and X-linked severe combined immunodeficiency4 due to a cryptic transcriptional splice site present within the insulator element that acted as a potent gene trap. Even when lentiviral vectors lack strong promoter and insulator elements, safety issues have been associated with the ablative preconditioning regimen needed for HSC transplant for sickle cell disease. In the clinical trial for treatment of sickle cell disease using LentiGlobin vector for ex vivo transfer of a modified anti-sickling beta-globin gene into patient HSCs, acute myeloid leukemias developed in 2 patients, apparently due to the busulfan conditioning regimen.5 As an alternative approach to using gene transfer vectors to introduce a new gene copy under the control of a foreign promoter while leaving a disease allele intact, gene correction using CRISPR (for clustered regularly interspaced short palindromic repeats)/Cas9 nuclease can be used to induce a double-strand DNA break at a mutated allele combined with a DNA donor template to mediate homology-directed repair of disease mutations while retaining normal regulation of gene expression. One such approach for correcting the sickle globin gene is currently undergoing a clinical trial, utilizing CRISPR/Cas9 combined with an adeno-associated virus vector to deliver a DNA template for repair of the beta-globin gene.6 However, such approaches do have the potential to damage the ability of HSCs to engraft long-term following ex vivo editing, due to the DNA damage response by the stem cells to both CRISPR/Cas9 and adeno-associated virus vectors.7 These issues highlight the need for improved methods of delivering gene therapy or gene repair vectors without inducing mutagenesis or impacting hematopoietic engraftment.
Li et al describe a gene therapy approach for sickle cell disease that could address many of the above issues by using gene editing of the sickle globin gene in HSCs in vivo by infusion of a prime editing vector in mouse bone marrow. Unlike CRISPR/Cas9 nuclease-based homology-directed repair using a coadministered DNA template, prime editing targets a DNA site using a catalytically impaired Cas9 nickase and delivers a DNA template for repair through a reverse-transcribed RNA sequence included in the target-specific prime editing guide RNA.8 The lack of nuclease activity greatly reduces the generation of unwanted insertions and deletions at the target site, making this approach safer than use of CRISPR/Cas9 nuclease, and the primer editing guide RNA delivers the repair template directly to the target site without the need for coadministration of a DNA template using an adeno-associated virus or other DNA vectors. Using this approach, Li et al report the achievement of therapeutic levels of beta-globin gene correction in mouse HSCs in vivo using a prime editing vector administered intravenously in mice. This level of correction was maintained in secondary transplants, demonstrating successful editing of long-term repopulating HSCs. However, achieving these therapeutic levels required in vivo selection with an alkylating agent for a drug-resistance gene present in the prime editing vector. Additionally, the authors demonstrate prime editing correction of human sickle cell disease patient HSCs ex vivo, with only low levels of unwanted insertions and deletions and no evidence of off-target effects in the edited cells.
This in vivo prime editing strategy still requires additional optimization to improve the overall gene editing efficiency to eliminate the need for in vivo selection with a potentially mutagenic alkylating agent. Additionally, the adenovirus vector used for delivery of the prime editor in this study could potentially induce an inflammatory response. Thus, replacement of the adenovirus vector with safer vectors might be desirable, such as virus-like particles for protein delivery9 or lipid nanoparticles for mRNA delivery.10 These vectors would have to be capable of mediating efficient delivery to HSCs without vector sequestration in unwanted tissues—although systemic or organic-targeted delivery of gene editing vectors may also be useful for disorders affecting nonhematopoietic tissues.
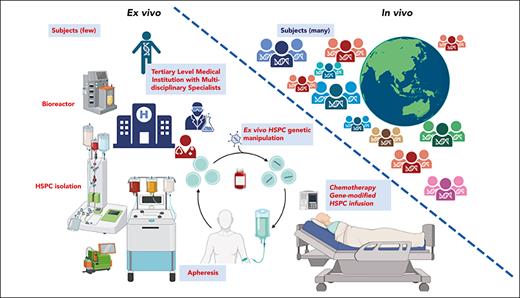
Despite the remaining technical issues involved in achieving therapeutic efficacy, in vivo prime editing holds the promise of lowering many of the barriers involved in the physical application of hematopoietic gene therapy technologies, particularly regarding the difficulties involved in HSC transplantation, complex ex vivo stem cell collection and manipulation, and cytoreductive conditioning. Further development of in vivo prime editing and base editing technologies could enable global dissemination of gene therapy treatments to developing countries and other regions that lack HSC transplant programs (see figure).
Ex vivo HSC gene therapy requires collection and purification of the patient’s HSCs and in vitro manipulation of the stem cells to induce proliferation, followed by gene therapy and finally transplantation. Typically, cytoreductive conditioning of the patient is required to make room for engrafting stem cells—manipulations that increase the costs of treatment and require facilities capable of performing stem cell transplants. In contrast, in vivo gene editing potentially could be performed by simple infusion of a suitable vector to deliver a prime editor to HSCs within the bone marrow, bypassing the need for stem cell transplantation and allowing for greater dissemination of gene therapy treatments globally. Figure created with BioRender.com.
Ex vivo HSC gene therapy requires collection and purification of the patient’s HSCs and in vitro manipulation of the stem cells to induce proliferation, followed by gene therapy and finally transplantation. Typically, cytoreductive conditioning of the patient is required to make room for engrafting stem cells—manipulations that increase the costs of treatment and require facilities capable of performing stem cell transplants. In contrast, in vivo gene editing potentially could be performed by simple infusion of a suitable vector to deliver a prime editor to HSCs within the bone marrow, bypassing the need for stem cell transplantation and allowing for greater dissemination of gene therapy treatments globally. Figure created with BioRender.com.
Conflict-of-interest disclosure: The authors declare no competing financial interests

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal