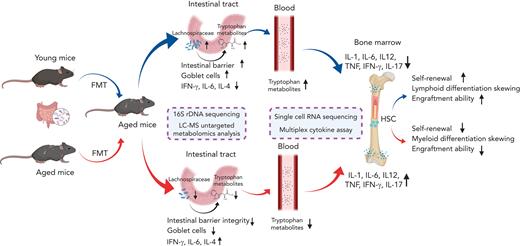
Key Points
FMT from young mice restored lymphoid differentiative potential and improved the number and engraftment ability of aged HSCs.
Lachnospiraceae and tryptophan-associated metabolites could improve both the phenotype and the reconstitution capacity of HSCs in aged mice.
Abstract
Hematopoietic stem cell (HSC) aging is accompanied by hematopoietic reconstitution dysfunction, including loss of regenerative and engraftment ability, myeloid differentiation bias, and elevated risks of hematopoietic malignancies. Gut microbiota, a key regulator of host health and immunity, has recently been reported to affect hematopoiesis. However, there is currently limited empirical evidence explaining the direct impact of gut microbiome on aging hematopoiesis. In this study, we performed fecal microbiota transplantation (FMT) from young mice to aged mice and observed a significant increment in lymphoid differentiation and decrease in myeloid differentiation in aged recipient mice. Furthermore, FMT from young mice rejuvenated aged HSCs with enhanced short-term and long-term hematopoietic repopulation capacity. Mechanistically, single-cell RNA sequencing deciphered that FMT from young mice mitigated inflammatory signals, upregulated the FoxO signaling pathway, and promoted lymphoid differentiation of HSCs during aging. Finally, integrated microbiome and metabolome analyses uncovered that FMT reshaped gut microbiota composition and metabolite landscape, and Lachnospiraceae and tryptophan-associated metabolites promoted the recovery of hematopoiesis and rejuvenated aged HSCs. Together, our study highlights the paramount importance of the gut microbiota in HSC aging and provides insights into therapeutic strategies for aging-related hematologic disorders.
Introduction
Hematopoietic stem cells (HSCs), characterized by long-term self-renewal ability and multilineage differentiation potential, are rare, multipotent precursors that reside at the apex of hematopoietic hierarchy. The senescence of HSCs is associated with hematopoietic reconstitution dysfunction, including loss of regenerative and engraftment ability,1,2 myeloid differentiation bias,3,4 as well as elevated risks of hematopoietic malignancies.5 These defects of aged HSCs have been attributed to accumulation of DNA damage,6 increasing levels of reactive oxygen species,7 and epigenetic and transcriptional alterations.8 For instance, the differentiation bias of aged HSCs is accompanied by the systemic reduction of genes involved in specifying lymphoid fate and upregulation of genes mediating myeloid differentiation.5 More recently, it has been shown through single-cell RNA sequencing (scRNA-seq) that murine HSCs at different ages present various responses to inflammatory stimulations with age-dependent inflammatory myeloid bias.9 Furthermore, epigenetic dysregulation in HSCs accumulates during aging, and in turn, leads to transcriptional alterations and the subsequent impaired cellular functions.10 Although accumulating evidence delineates the molecular mechanisms underlying HSC aging, delaying or rejuvenating the senescence of HSCs still remains challenging. Therefore, new strategies to improve the function of aged HSCs need to be developed further.
Gut microbiota, the microbial communities that inhabit the gastrointestinal tract, has attracted wide attention because of its fundamental roles in host health. In addition to absorbing nutrients and maintaining homeostasis in the intestine, the gut microbiota delivers signals to distant organs and regulates systemic immune function and metabolism.11,12 Microbial dysbiosis in the intestine is associated with numerous diseases including allergic disorders, cancers, and cardiovascular diseases.13-15 Notably, emerging studies have implicated the gut microbiota as a major regulator of both normal and aberrant hematopoiesis.16-20 Depletion of the gut microbiota by antibiotics severely disturbs the murine hematopoietic homeostasis, whereas fecal microbiota transplantation (FMT) from nonantibiotic-treated mice could partially abrogate the detrimental effects of antibiotics on hematopoiesis.16 Kovtonyuk et al recently demonstrated that gut microbiome components contributed to a higher level of interleukin 1 (IL-1) in aged mice and served as a vital driver of the aging-dependent myeloid differentiation bias of HSCs.18 Furthermore, the gut microbiota modulated macrophage functions via short-chain fatty acid (SCFA) production, which in turn acted on the distribution of iron and regulated HSCs fate decision.19 Moreover, technological advancement in liquid chromatography and mass spectrometry (LC-MS) allows the analysis of gut metabolites, which provides unprecedented insights into the causative relationship between the microbiota, metabolites, and host. For example, SCFAs were identified as key molecules produced by colonic microbial fermentation, which contributed to the homeostasis of immune cells and the functions of hematopoietic cells in the host.21 However, the roles and mechanisms of action of the gut microbiota in HSC aging, and whether modulation of the gut microbiota could rejuvenate aged HSCs remain largely unknown.
In this study, we performed FMT from young mice to aged mice and observed restoration of hematopoiesis in aged mice. We also performed scRNA-seq, 16S ribosomal RNA (rRNA) gene sequencing and LC-MS untargeted metabolomic analysis to excavate the mechanisms underlying the beneficial effects of FMT.
Methods
Animals
Young (7-8 weeks) and aged (20-24 months) CD45.2 or CD45.1 C57BL/6 mice were employed for all experiments of this study. Mice were housed under specific pathogen-free conditions in the Laboratory Animal Center of Zhejiang University. All animal experiments were approved by the Zhejiang University animal ethics committee.
FMT
Before FMT, mice were orally gavaged with 100 μL antibiotic cocktails for 7 days, containing neomycin (0.5 g/L), metronidazole (1 g/L), vancomycin (0.5 g/L), and ampicillin (1 g/L). Afterward, mice were orally gavaged with 100 μL microbiota suspension, daily for 4 weeks. For the microbiota suspension preparation, young or aged mice were individually placed in clean cages daily and fecal pellets were collected within 30 minutes. Fresh fecal pellets (50 mg) were suspended in 1.5 mL phosphate-buffered saline.
scRNA-seq
Whole bone marrow (BM) cells, immature hematopoietic cells (Lin−), and progenitor cells (Lin− cKit+) were collected by flow cytometric sorting and mixed at a 1:1:4 ratio for scRNA-seq sample preparation. Then scRNA-seq data were generated using 10X Genomics Chromium Single Cell 3′ kits.
Fecal microbiota and metabolite evaluation
Mice were individually placed in clean cages for feces collection. Fresh fecal samples were collected into sterile cryopreservation tubes, immediately frozen in liquid nitrogen, and stored at −80°C. LC-MS untargeted metabolomic analysis and 16S rRNA gene sequencing were performed as previously reported.22
Statistical analysis
Statistical analysis was conducted by using GraphPad. Comparisons between 2 groups were determined by using a 2-tailed Student t test. Comparison of multiple groups were determined by using a one-way analysis of variance with the Dunnett multiple comparisons test. All experiments in this study were repeated 2 or 3 times independently, and representative data are shown. Data with P < .05 were considered statistically significant.
Results
FMT from young mice ameliorated defective phenotype of aged HSCs
To evaluate the influence of the gut microbiota on aging hematopoiesis, the gut microbiota from healthy young donor mice or aged donor mice were transplanted into aged recipient mice (FMT-YA and FMT-AA group, respectively) (Figure 1A). Antibiotics were used 1 week before FMT to remove the original gut microbiota from recipient mice, which facilitated the colonization of newly inhabited bacteria. Aged mice treated with antibiotics (antibiotics group), normal control aged mice (aged group), and normal control young mice (young group) served as experimental controls.
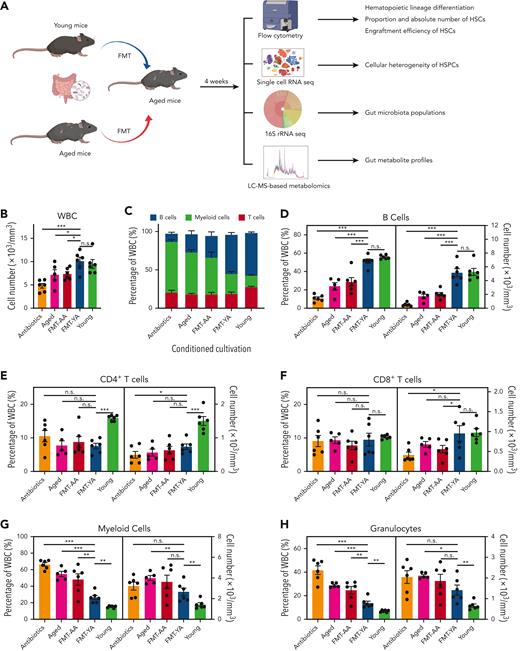
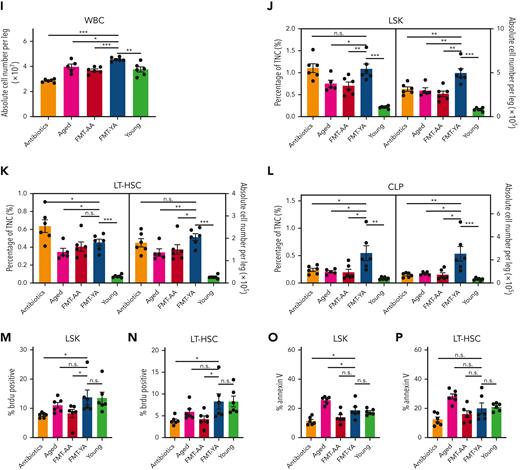
Transplantation of young gut microbiota restored lymphoid/myeloid cell ratio and increased the proportion and absolute number of HSCs in aged mice. (A) A flowchart of animal treatments. (B) WBC concentration in PB, as determined by an automated hematologic cell counter, in mice in the antibiotics group, aged group, FMT-AA group, FMT-YA group, and the young control group. (C) Hematopoietic lineage differentiation in PB. (D-H) Proportions and concentrations of B cells, CD4+ T cells, CD8+ T cells, myeloid cells, and granulocytes in PB. (I) Count of WBCs in BM. (J-L) Proportions and absolute numbers of HSPCs, LT-HSCs, and common lymphoid progenitors (CLPs) in BM. (M-N) 5-bromo-2′-deoxyuridine (BrdU) incorporation was detected by anti-BrdU antibody in HSPCs and LT-HSCs. (O-P) Apoptosis was measured by annexin V staining in HSPCs and LT-HSCs. Each point represents data from a single mouse (n = 6 per group). Graphs show mean ± standard error of the mean (SEM), with statistical significance determined by Student t test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. n.s., nonsignificant; TNC, total nucleated cell.
Transplantation of young gut microbiota restored lymphoid/myeloid cell ratio and increased the proportion and absolute number of HSCs in aged mice. (A) A flowchart of animal treatments. (B) WBC concentration in PB, as determined by an automated hematologic cell counter, in mice in the antibiotics group, aged group, FMT-AA group, FMT-YA group, and the young control group. (C) Hematopoietic lineage differentiation in PB. (D-H) Proportions and concentrations of B cells, CD4+ T cells, CD8+ T cells, myeloid cells, and granulocytes in PB. (I) Count of WBCs in BM. (J-L) Proportions and absolute numbers of HSPCs, LT-HSCs, and common lymphoid progenitors (CLPs) in BM. (M-N) 5-bromo-2′-deoxyuridine (BrdU) incorporation was detected by anti-BrdU antibody in HSPCs and LT-HSCs. (O-P) Apoptosis was measured by annexin V staining in HSPCs and LT-HSCs. Each point represents data from a single mouse (n = 6 per group). Graphs show mean ± standard error of the mean (SEM), with statistical significance determined by Student t test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. n.s., nonsignificant; TNC, total nucleated cell.
We initially investigated the impact of FMT on peripheral blood (PB). The number of white blood cells (WBCs) in PB was elevated in FMT-YA mice (Figure 1B). Particularly, the proportion and absolute number of B cells were significantly increased in the FMT-YA group and was similar to that in the young control mice (Figure 1C-D; supplemental Figure 1A, available on the Blood website). Although no differences in CD4+ T cells were observed within FMT groups, the number of CD8+ T cells was upregulated in FMT-YA mice (Figure 1E-F). Furthermore, the gut microbiota from young mice resulted in diminished myeloid cells, especially granulocytes (Figure 1G-H). Consistently, the restoration of lymphoid differentiation in FMT-YA was also observed in BM (supplemental Figure 1B).
To further assess the effects of FMT on hematopoietic precursors, the proportion and absolute number of hematopoietic stem and progenitor cells (HSPCs) in BM were examined. FMT-YA mice displayed an increased proportion and absolute numbers of LSKs (Lin− Sca-1+cKit+); long-term HSCs (LT-HSCs), short-term HSCs, and multipotent progenitors (MPPs) were significantly elevated (Figure 1I-K; supplemental Figure 1C-E). Concordant with the enhancement of mature lymphocytes in PB, the proportion and number of common lymphoid progenitors were markedly increased in FMT-YA mice (Figure 1L; supplemental Figure 1F-H). Moreover, 5-bromo-2′-deoxyuridine incorporation assay showed an increase of newly generated HSPCs and LT-HSCs in FMT-YA mice, whereas apoptosis of hematopoietic precursors was not affected by FMT (Figure 1M-P; supplemental Figure 1I-L). In summary, FMT from young mice could restore lymphoid-to-myeloid cell ratio and increase the proportion and absolute number of HSCs.
FMT from young mice restored the reconstitution capacity of aged HSCs
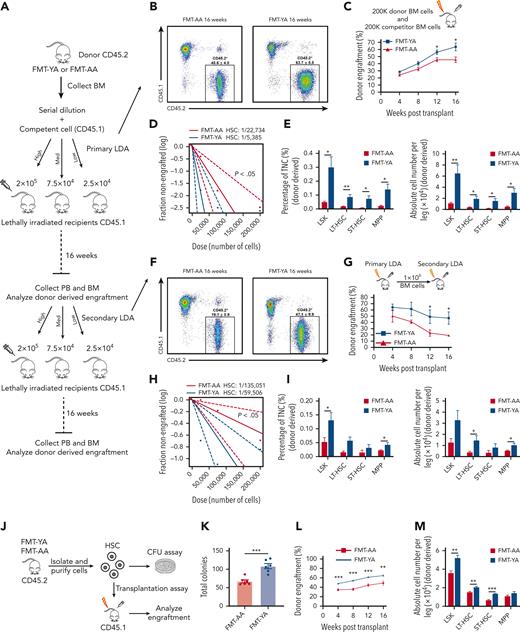
To demonstrate the effect of young gut microbiota on the reconstitution ability of aged HSCs, we carried out a limiting-dilution competitive repopulation unit (CRU) assay by transplanting FMT-YA or FMT-AA BM cells (2.5 × 104, 7.5 × 104, or 2 × 105), together with 2 × 105 recipient BM cells (CD45.1), into lethally irradiated recipient mice (Figure 2A). FMT-YA BM cells displayed a significantly elevated engraftment rate at 16 weeks after transplantation compared with FMT-AA BM cells (Figure 2B-C), and the CRU assay showed a 4.2-fold increase in functional HSCs in FMT-YA mice (Figure 2D). Furthermore, recipient mice that received transplantation with FMT-YA BM cells exhibited a marked increase in both frequency and absolute number of donor-derived HSPCs and committed progenitors (Figure 2E; supplemental Figure 2A-B), whereas no difference in lineage biases was observed between recipients of FMT-YA or FMT-AA cells (supplemental Figure 2C). Next, secondary transplantation was performed in the fourth month after primary transplantation to evaluate the long-term repopulation ability. FMT-YA cells displayed higher engraftment efficiency (Figure 2F-G). Notably, CRUs increased 2.6 fold in FMT-YA cells with raised numbers of LT-HSCs and MPPs in recipients (Figure 2H-I; supplemental Figure 2D-F). Clonogenic assay and further transplantation assay were applied to validate the stemness of purified LT-HSC, which confirmed that cellular function of HSCs was enhanced by FMT from young mice (Figure 2J-M; supplemental Figure 2G-I). Together, FMT from young mice rejuvenated aged HSCs with enhanced short-term and long-term hematopoietic repopulation capacity.
Transplantation of young gut microbiota increased functional HSCs in mice. (A) Flowchart for limiting-dilution transplantation assay (LDA) to determine the frequency of functional HSCs in FMT-AA mice and FMT-YA mice. (B-C) Representative flow cytometry plots and line graph of donor-derived CD45.2+ reconstitution in CD45.1+ mice receiving the highest cell doses at 16 weeks after transplantation in primary LDA. (D) HSC frequency determined by primary LDA. (E) Frequency in TNCs and absolute cell number of donor-derived HSPCs in BM from recipient mice in primary LDA. (F-G) Representative flow cytometry plots and line graph of donor-derived CD45.2+ reconstitution in CD45.1+ mice receiving the highest cell doses at 16 weeks after transplantation in secondary LDA. (H) HSC frequency determined by secondary LDA. (I) Frequency in TNCs and absolute cell number of donor-derived HSPCs in BM from recipient mice in secondary LDA. (J) Flowchart for clonogenic assay and transplantation assay of purified LT-HSCs. (K) Number of total colonies measured for LT-HSCs in FMT-AA mice and FMT-YA mice in methylcellulose. (L) Line graph of donor-derived CD45.2+ reconstitution in CD45.1+ mice at 16 weeks after HSC transplantation. (M) Absolute cell numbers of donor-derived HSPCs in BM from recipient mice in HSC transplantation assay. Graphs show mean ± SEM, with statistical significance determined by Student t test (n = 6 per group). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. Med, medium; ST-HSC, short-term HSC.
Transplantation of young gut microbiota increased functional HSCs in mice. (A) Flowchart for limiting-dilution transplantation assay (LDA) to determine the frequency of functional HSCs in FMT-AA mice and FMT-YA mice. (B-C) Representative flow cytometry plots and line graph of donor-derived CD45.2+ reconstitution in CD45.1+ mice receiving the highest cell doses at 16 weeks after transplantation in primary LDA. (D) HSC frequency determined by primary LDA. (E) Frequency in TNCs and absolute cell number of donor-derived HSPCs in BM from recipient mice in primary LDA. (F-G) Representative flow cytometry plots and line graph of donor-derived CD45.2+ reconstitution in CD45.1+ mice receiving the highest cell doses at 16 weeks after transplantation in secondary LDA. (H) HSC frequency determined by secondary LDA. (I) Frequency in TNCs and absolute cell number of donor-derived HSPCs in BM from recipient mice in secondary LDA. (J) Flowchart for clonogenic assay and transplantation assay of purified LT-HSCs. (K) Number of total colonies measured for LT-HSCs in FMT-AA mice and FMT-YA mice in methylcellulose. (L) Line graph of donor-derived CD45.2+ reconstitution in CD45.1+ mice at 16 weeks after HSC transplantation. (M) Absolute cell numbers of donor-derived HSPCs in BM from recipient mice in HSC transplantation assay. Graphs show mean ± SEM, with statistical significance determined by Student t test (n = 6 per group). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001. Med, medium; ST-HSC, short-term HSC.
scRNA-seq revealed that FMT from young mice reduced inflammation, activated the FoxO pathway, and promoted lymphoid differentiation in aged LT-HSCs
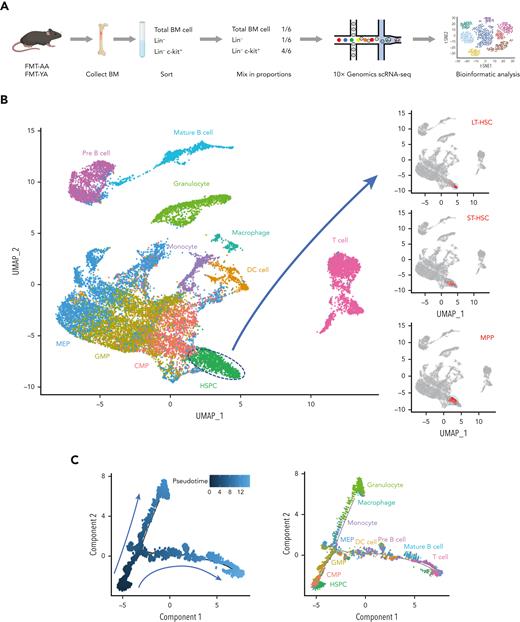
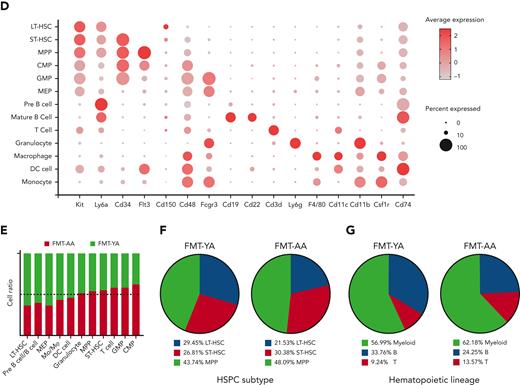
To systematically decipher the impact of FMT on the transcriptome levels of HSCs in aged mice, we performed scRNA-seq of BM cells from FMT-YA and FMT-AA mice. Whole BM cells, immature hematopoietic cells (Lin−), and progenitor cells (Lin− cKit+) were collected by flow cytometric sorting and admixed at a 1:1:4 ratio and then subjected to the 10X Genomics Chromium capture platform (Figure 3A; supplemental Figure 3A). We obtained a total of 21 715 high-quality cells with an average of 3014 genes per cell (11 056 FMT-YA cells and 10 659 FMT-AA cells after quality control) (supplemental Figure 3B-C). Twenty-eight unbiased cell clusters were identified and visualized by uniform manifold approximation and projection, spanning from HSPCs to mature hematopoietic cells. Cell type labels were assigned to each cluster based on the canonical marker genes of previous reports:23,24 LT-HSCs (Ly6a+ cKit+ CD34− Flt3− CD150+ CD48−), short-term HSCs (Ly6a+ cKit+ CD34+ Flt3− CD150− CD48−), MPPs (Ly6a+ cKit+ CD34+ Flt3+ CD150− CD48+), common myeloid progenitors (cKit+ CD34+ Cebpa+ Ly6a− Fcgr3−), granulocyte/monocyte progenitors (cKit+ CD34+ Ly6a− Fcgr3+), megakaryocyte/erythroid progenitors (cKit+ CD34− CD71+ Ly6a−), granulocytes (CD11b+ Ly6g+), macrophages (F4/80+ CD11b+), dendritic cells (CD11c+ CD83+ CD74+), monocytes (CD115+ Ly86+ Ly6c2+), pre–B cells (Igkc+ Ighm+), B cells (CD19+ Fcrla+), and T cells (CD3d+) (Figure 3B-D; supplemental Figure 3D-E). Notably, both uniform manifold approximation and projection and pseudotime analyses showed LT-HSCs at the apex of the hematopoietic hierarchy, which were differentiated into myeloid and lymphoid lineage cells, supporting the high quality of our scRNA-seq data (Figure 3B-C). Consistent with the flow cytometry analyses, FMT-YA displayed a significant increase in LT-HSCs among HSPC subtypes, whereas B cells accounted for the higher percentage of hematopoietic lineages (Figure 3E-G).
A transcriptome-wide landscape of hematopoiesis after FMT by scRNA-seq. (A) Flowchart for cell collection and data analysis in scRNA-seq. BM cells were harvested from FMT-AA mice and FMT-YA mice. Whole BM cells, immature hematopoietic cells (Lin−), and hematopoietic progenitor cells (Lin− cKit+) were collected by flow cytometric sorting and admixed at a 1:1:4 ratio and then subjected to 10X Genomics Chromium capture platform. (B) Uniform manifold approximation and projection (UMAP) plot showing the clustering results of hematopoietic cells. (C) Pseudotime trajectory analysis of hematopoiesis from HSCs to mature hematopoietic cells. (D) Bubble plot showing the expression of key marker genes in different cell clusters. (E) Bar graph showing the cell ratios of hematopoietic cells in FMT-YA and FMT-AA mice, based on scRNA-seq. (F-G) Pie charts showing the relative abundance of HSPC subpopulations and hematopoietic lineage differentiation in FMT-YA and FMT-AA mice. DC, dendritic cell; CMP, common myeloid progenitor; GMP, granulocyte/monocyte progenitor; MEP, megakaryocyte/erythroid progenitor; Mo, monocyte.
A transcriptome-wide landscape of hematopoiesis after FMT by scRNA-seq. (A) Flowchart for cell collection and data analysis in scRNA-seq. BM cells were harvested from FMT-AA mice and FMT-YA mice. Whole BM cells, immature hematopoietic cells (Lin−), and hematopoietic progenitor cells (Lin− cKit+) were collected by flow cytometric sorting and admixed at a 1:1:4 ratio and then subjected to 10X Genomics Chromium capture platform. (B) Uniform manifold approximation and projection (UMAP) plot showing the clustering results of hematopoietic cells. (C) Pseudotime trajectory analysis of hematopoiesis from HSCs to mature hematopoietic cells. (D) Bubble plot showing the expression of key marker genes in different cell clusters. (E) Bar graph showing the cell ratios of hematopoietic cells in FMT-YA and FMT-AA mice, based on scRNA-seq. (F-G) Pie charts showing the relative abundance of HSPC subpopulations and hematopoietic lineage differentiation in FMT-YA and FMT-AA mice. DC, dendritic cell; CMP, common myeloid progenitor; GMP, granulocyte/monocyte progenitor; MEP, megakaryocyte/erythroid progenitor; Mo, monocyte.
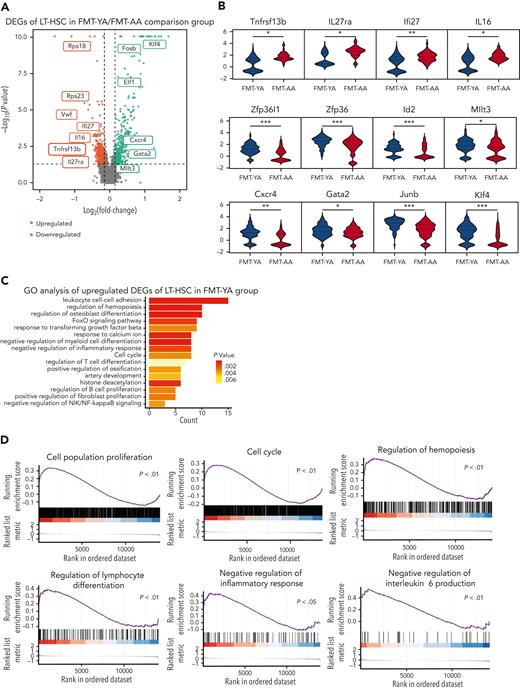
We then examined transcriptomic alterations in HSCs and identified a total of 829 differentially expressed genes (DEGs) between FMT-YA and FMT-AA (Figure 4A; supplemental Table 1). The expression of inflammation-related genes was downregulated in FMT-YA (Tnfrsf13b, Il27ra, Il16, and Ifi27), whereas genes involved in HSC self-renewal (Gata2, Mllt3, and Cxcr4) and lymphoid fate determination (Zpf36l1, Zpf36, and Id2) were upregulated in FMT-YA (Figure 4B). Compared with a recently published meta-analysis of aged HSCs,25 some common aging signatures were significantly downregulated after FMT from young mice (including Vwf, Ehd3, Aspa, Cysltr2, Nt5c3, and Itga6), which demonstrated that young microbiota could partially restore the aberrant transcriptome of aged HSCs. Further gene ontology enrichment analysis suggested that pathways related to cell population proliferation, regulation of hemopoiesis, negative regulation of inflammatory response, as well as the FoxO signaling pathway were enriched in LT-HSCs from FMT-YA mice (Figure 4C-D; supplemental Figure 4A-C). Of note, the FoxO signaling pathway reportedly protects HSCs from DNA damage and ameliorates HSC aging.26-28 Immunofluorescent staining confirmed that LT-HSCs had high-level expression of FoxO family transcription factors in FMT-YA, and γH2AX staining showed that the indicator of damaged DNA in HSCs decreased in FMT-YA mice (supplemental Figure 4D-F). Besides, inflammatory pathways were downregulated in other hematopoietic cells of FMT-YA mice, especially in granulocytes, monocytes, and macrophages (supplemental Figure 4G), which was consistent with previous reports that inflammatory cytokines secreted by mature hematopoietic cells might contribute to the senescent phenotype of HSCs.18,29,30
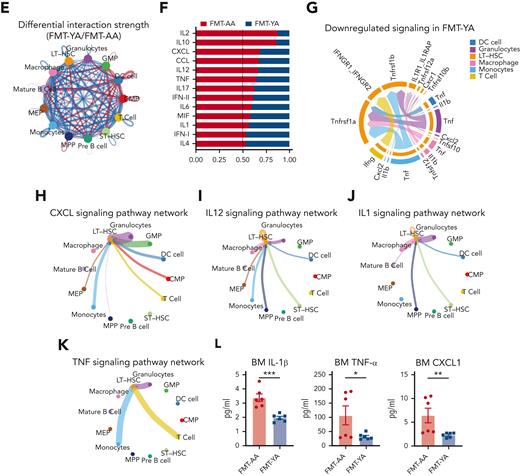
FMT from young mice reduced inflammation and promoted lymphoid differentiation in aged LT-HSCs. (A) Volcano plot for DEGs in LT-HSCs. (B) Violin plots showing the expression of inflammation-related genes, stemness-related genes, and lymphoid-specific genes. (C) Representative gene ontology (GO) analysis results enriched in upregulated DEGs of LT-HSCs in the FMT-YA group. (D) Representative gene set enrichment analysis enrichment plots of GO terms in LT-HSCs. (E) Circle plot showing differential interaction strength in the cell-to-cell communications between FMT-YA and FMT-AA. Blue-colored edges represent decreased signaling in the FMT-YA data set and red-colored edges represent increased signaling in the FMT-YA data set. (F) Enrichment analysis results of differential ligand-receptor pairs targeting LT-HSCs between FMT-YA and FMT-AA. (G) Chord diagram showing downregulated signaling ligand-receptor pairs targeting LT-HSCs in FMT-YA. (H-K) Circle plot showing the source of inflammatory cytokines. Edge weights are proportional to the interaction strength. (L) Bar graphs showing the BM serum concentrations of IL-1β, TNF-α, and CXCL1. Graphs show mean ± SEM (n = 6 per group), with statistical significance determined by Student t test (n = 6 per group). ∗P < .05, ∗∗P < .01.
FMT from young mice reduced inflammation and promoted lymphoid differentiation in aged LT-HSCs. (A) Volcano plot for DEGs in LT-HSCs. (B) Violin plots showing the expression of inflammation-related genes, stemness-related genes, and lymphoid-specific genes. (C) Representative gene ontology (GO) analysis results enriched in upregulated DEGs of LT-HSCs in the FMT-YA group. (D) Representative gene set enrichment analysis enrichment plots of GO terms in LT-HSCs. (E) Circle plot showing differential interaction strength in the cell-to-cell communications between FMT-YA and FMT-AA. Blue-colored edges represent decreased signaling in the FMT-YA data set and red-colored edges represent increased signaling in the FMT-YA data set. (F) Enrichment analysis results of differential ligand-receptor pairs targeting LT-HSCs between FMT-YA and FMT-AA. (G) Chord diagram showing downregulated signaling ligand-receptor pairs targeting LT-HSCs in FMT-YA. (H-K) Circle plot showing the source of inflammatory cytokines. Edge weights are proportional to the interaction strength. (L) Bar graphs showing the BM serum concentrations of IL-1β, TNF-α, and CXCL1. Graphs show mean ± SEM (n = 6 per group), with statistical significance determined by Student t test (n = 6 per group). ∗P < .05, ∗∗P < .01.
To define the cell-to-cell communications between HSCs and other hematopoietic cells, we inferred the biologically significant cell-to-cell communication using Cellchat31 and observed an overall reduction of cell-to-cell interactions in FMT-YA mice (Figure 4E). In particular, decreased interactions involving LT-HSCs in FMT-YA mice were mainly in inflammatory cytokines pathways, for example, IL-2, tumor necrosis factor (TNF), IL-17, and IL-6 (Figure 4F-G). We then focused on the network of several inflammatory pathways among CXCL, IL-12, IL-1, and TNF signaling, and observed strong interactions between myeloid cells and LT-HSCs, which suggested that young gut microbiota might decrease the secretion of inflammatory cytokines in myeloid cells (Figure 4H-K; supplemental Figure 4I-J). To validate this hypothesis, we determined the concentrations of cytokines in BM serum, and found that IL-1β, TNF-α, and CXCL1 were downregulated in FMT-YA mice compared with FMT-AA mice (Figure 4L). As for the upregulated DEGs in FMT-YA, gene ontology analysis indicated that the gut microbiota from young mice promoted immune cell proliferation and restored immunocompetence (supplemental Figure 4H). Consequently, our scRNA-seq data depicted a transcriptome-wide landscape of the BM immune microenvironment after FMT, indicating that the gut microbiota from young mice reduced inflammation and reinvigorated lymphoid differentiation in aged LT-HSCs.
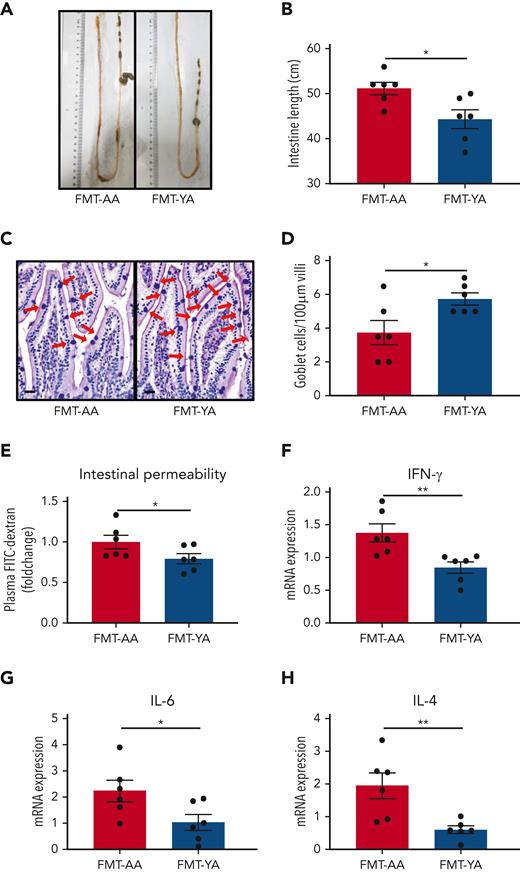
FMT from young mice protected intestinal barrier integrity in aged mice
Because intestinal tracts are the main location where the microbiota colonizes and interacts with the host, we sought to investigate the morphological and biological characteristics of the intestinal barrier. A recent study reported an increase in intestinal length and surface area in germ-free mice that received transplantation with fecal microbiota from old donor mice.32 Similarly, FMT-AA mice presented longer intestinal length compared with FMT-YA mice (Figure 5A-B). Goblet cells are a specialized type of epithelial cell that secrete mucins for cell lubrication and protection.33 Quantitative analysis of goblet cells by hematoxylin and eosin and Alcian Blue PAS staining showed more goblet cells in simple epithelia in FMT-YA mice (Figure 5C-D). Moreover, assessment of circulating fluorescein isothiocyanate–labeled dextran showed that the intestinal barrier integrity was improved with treatment with young gut microbiota (Figure 5E). A previous study has shown that dysfunction of the intestinal barrier could result in bacterial translocation and an increase in inflammatory signals, which in turn affect myeloproliferation and hematopoietic malignancies.34 Thus, the improvement of intestinal barrier integrity may be a vital link in rejuvenating aged HSCs with young microbiota. Furthermore, we observed a lower expression of intestinal inflammatory markers in FMT-YA mice, including interferon gamma, IL-6, and IL-4 (Figure 5F-H). Taken together, these data suggest that young gut microbiota was supportive and protective for intestinal barrier integrity in aged mice.
Young gut microbiota reduced inflammation and protected intestinal barriers in aged mice. (A) Representative images showing the intestinal length of FMT-YA and FMT-AA mice. (B) Bar graphs showing the intestinal lengths of FMT-YA and FMT-AA mice. (C) Representative images showing the number of goblet cells by Acian Blue PAS staining. Scale bars, 20 μm. (D) Bar graphs showing the number of goblet cells from FMT-YA and FMT-AA mice. (E) The intestinal permeability of FMT-YA and FMT-AA mice measured by fluorescein isothiocyanate (FITC)–dextran in blood. Data were normalized to aged FMT-AA controls. (F-H) The expression of interferon gamma (IFN-γ), IL-6, and IL-4 in intestinal tissue by quantitative reverse transcription polymerase chain reaction. Graphs show mean ± SEM, with statistical significance determined by Student t test (n = 6 per group). ∗P < .05, ∗∗P < .01. mRNA, messenger RNA.
Young gut microbiota reduced inflammation and protected intestinal barriers in aged mice. (A) Representative images showing the intestinal length of FMT-YA and FMT-AA mice. (B) Bar graphs showing the intestinal lengths of FMT-YA and FMT-AA mice. (C) Representative images showing the number of goblet cells by Acian Blue PAS staining. Scale bars, 20 μm. (D) Bar graphs showing the number of goblet cells from FMT-YA and FMT-AA mice. (E) The intestinal permeability of FMT-YA and FMT-AA mice measured by fluorescein isothiocyanate (FITC)–dextran in blood. Data were normalized to aged FMT-AA controls. (F-H) The expression of interferon gamma (IFN-γ), IL-6, and IL-4 in intestinal tissue by quantitative reverse transcription polymerase chain reaction. Graphs show mean ± SEM, with statistical significance determined by Student t test (n = 6 per group). ∗P < .05, ∗∗P < .01. mRNA, messenger RNA.
Lachnospiraceae restored defective HSC phenotype in aged mice
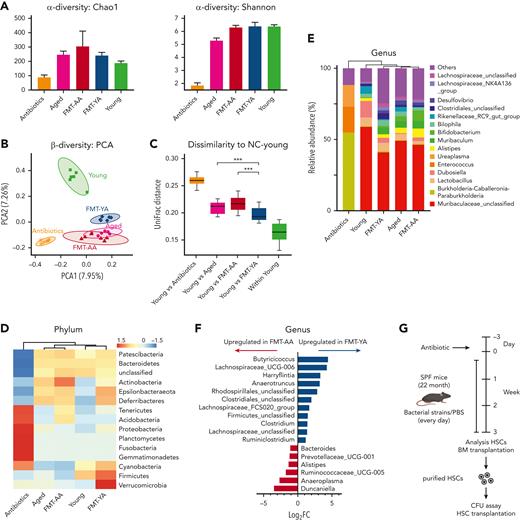
To address the relevance of the gut microbiome in HSC aging, we employed 16S rRNA gene sequencing to analyze the bacterial taxonomic composition after FMT. α-Diversity revealed that FMT-AA mice exhibited higher bacterial richness, corresponding to the abundance trend of their donors (Figure 6A; supplemental Figure 5A). In β-diversity analysis, principal component analysis revealed notable differences in the microbial landscape between FMT-YA and FMT-AA mice (Figure 6B). Further quantitative analysis by UniFrac dissimilarity distance supported that the microbiome composition of FMT-YA mice were similar to that of young donors (Figure 6C), confirming the successful implementation of the FMT strategy.
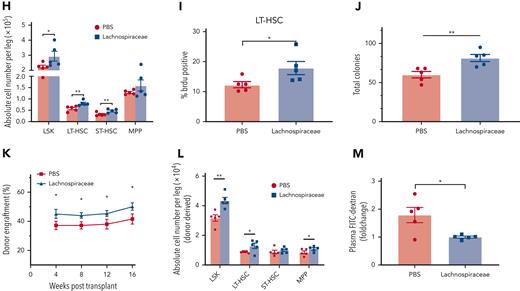
FMT effectively modulated the gut microbiome in the recipient aged mice. (A) α-diversity (Chao1 index and Shannon index) of bacterial communities in antibiotics-treated, aged, FMT-AA, FMT-YA, and young mice (n = 6 per group). (B-C) Principal component analysis (PCA) plot and UniFrac distance of microbial composition (n = 6 per group). (D) Average relative abundance of prevalent microbiota at the phylum level among the 5 groups. (E) Average relative abundance of prevalent microbiota at the genus level among the 5 groups (n = 6 per group). (F) Bar graph showing the differential microbiota between FMT-AA and FMT-YA mice at the gene level (n = 6 per group). (G) The flowchart of microbiota interventions. Specific pathogen-free (SPF) aged mice were given phosphate-buffered saline (PBS), Lachnospiraceae, or Clostridium by gavage for 4 weeks, after treatment with antibiotics for 3 days. Then, colony-forming unit (CFU) assay and transplantation assay were performed to assess the cellular function of purified LT-HSCs. (H) Absolute numbers of HSPCs in BM in PBS group vs Lachnospiraceae group (n = 5 per group). (I) BrdU incorporation in LT-HSCs (n = 5 per group). (J) Number of total colonies measured for LT-HSCs in methylcellulose (n = 5 per group). (K) Donor-derived CD45.2+ reconstitution in CD45.1+ mice at 16 weeks after HSC transplantation (n = 5 per group). (L) Absolute cell number of donor-derived HSPCs in BM from recipient mice in HSC transplantation assay (n = 5 per group). (M) Intestinal permeability measured by FITC-dextran in blood (n = 5 per group). Graphs show mean ± SEM, with statistical significance determined by Student t test. ∗P < .05, ∗∗P < .01.
FMT effectively modulated the gut microbiome in the recipient aged mice. (A) α-diversity (Chao1 index and Shannon index) of bacterial communities in antibiotics-treated, aged, FMT-AA, FMT-YA, and young mice (n = 6 per group). (B-C) Principal component analysis (PCA) plot and UniFrac distance of microbial composition (n = 6 per group). (D) Average relative abundance of prevalent microbiota at the phylum level among the 5 groups. (E) Average relative abundance of prevalent microbiota at the genus level among the 5 groups (n = 6 per group). (F) Bar graph showing the differential microbiota between FMT-AA and FMT-YA mice at the gene level (n = 6 per group). (G) The flowchart of microbiota interventions. Specific pathogen-free (SPF) aged mice were given phosphate-buffered saline (PBS), Lachnospiraceae, or Clostridium by gavage for 4 weeks, after treatment with antibiotics for 3 days. Then, colony-forming unit (CFU) assay and transplantation assay were performed to assess the cellular function of purified LT-HSCs. (H) Absolute numbers of HSPCs in BM in PBS group vs Lachnospiraceae group (n = 5 per group). (I) BrdU incorporation in LT-HSCs (n = 5 per group). (J) Number of total colonies measured for LT-HSCs in methylcellulose (n = 5 per group). (K) Donor-derived CD45.2+ reconstitution in CD45.1+ mice at 16 weeks after HSC transplantation (n = 5 per group). (L) Absolute cell number of donor-derived HSPCs in BM from recipient mice in HSC transplantation assay (n = 5 per group). (M) Intestinal permeability measured by FITC-dextran in blood (n = 5 per group). Graphs show mean ± SEM, with statistical significance determined by Student t test. ∗P < .05, ∗∗P < .01.
The heatmap at phylum level combined with clustering analysis showed similar microbiome compositions among samples of the recipient mice compared with their corresponding donors (Figure 6D; supplemental Figure 5B-C). Notably, Cyanobacteria and Firmicutes were enriched in the gut microbiota of FMT-YA mice and young mice, whereas Actinobacteria were enriched in FMT-AA mice and aged mice. At genus level, FMT-YA mice exhibited increments in Butyricicoccus, Lachnospiraceae, and Clostridium (Figure 6E-F; supplemental Figure 5D; supplemental Table 2). Furthermore, the abundance of Bacteroides, Alistipes, and Anaeroplasma was decreased in FMT-YA mice. Together, these findings indicated that FMT from young mice effectively modulated the gut microbiome in recipient aged mice.
To explore which specific bacterial taxa is responsible for the restoration of HSC defects in aged mice, we treated specific pathogen-free aged mice with antibiotics for 3 days, and then administrated Lachnospiraceae or Clostridium, 2 types of bacteria enriched in FMT-YA group, by daily oral gavage for 4 weeks (Figure 6G). Mice gavaged with Lachnospiraceae displayed an increased number of WBCs in BM, and elevated proportion and absolute number of HSPCs (Figure 6H; supplemental Figure 5E-H). Furthermore, 5-bromo-2′-deoxyuridine incorporation assay showed an increase in newly generated HSCs in mice treated with Lachnospiraceae (Figure 6I), and competitive BM transplantation assay indicated that Lachnospiraceae significantly improved engraftment efficiency of aged BM cells (supplemental Figure 5G). Further assessment of purified LT-HSCs by clonogenic assay and transplantation assay confirmed that cellular function of LT-HSCs was enhanced by Lachnospiraceae (Figure 6J-L; supplemental Figure 5H-J). Lastly, supplementation with Lachnospiraceae enhanced the intestinal barrier integrity (Figure 6M). Conversely, no significant advantages were observed in HSPCs in mice treated with Clostridium (supplemental Figure 5K-5L). Overall, these data implied that Lachnospiraceae were the key factor in the restoration of defective HSC phenotype in aged mice.
Supplement with tryptophan-associated metabolites improved engraftment efficiency of aged HSCs
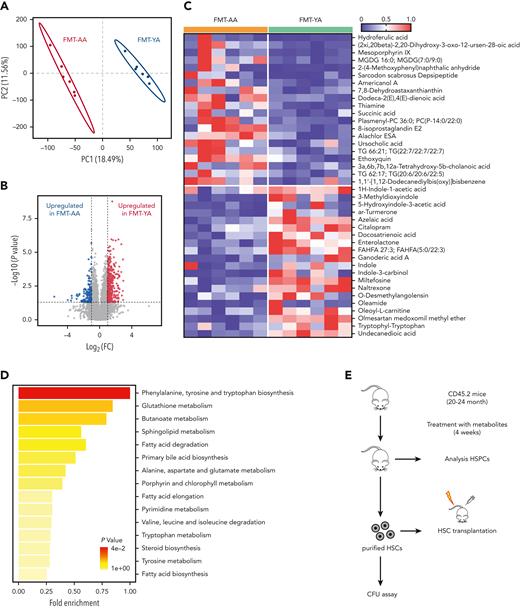
Many microbiota-derived metabolites play vital roles in the regulation of host inflammation and hematopoiesis via the bloodstream.21 To identify the microbiota-derived metabolites responsible for restoration of aging hematopoiesis, LC-MS untargeted metabolomic analysis of fecal samples was performed in mice upon FMT. Principal component analysis indicated that fecal samples of FMT-YA displayed distinct metabolite profiles compared with the FMT-AA group (Figure 7A-B). Specifically, tryptophan-associated metabolites, including tryptophan, indole-1-acetic acid (IAA), and indole and indole-3-carbinol (I3C), were significantly upregulated in FMT-YA mice (Figure 7C). Upregulation of tryptophan-associated metabolites in FMT-YA mice was also observed in PB and BM plasma (supplemental Figure 6A-B). Of note, indole and indole derivatives could prevent oxidative stress and reduce inflammatory response in vitro and in vivo.35,36 We then determined the enriched pathways of these metabolites by Kyoto Encyclopedia of Genes and Genomes analysis. Pathways related to various amino acids were enriched in FMT-YA mice, especially in phenylalanine, tyrosine, and tryptophan-related metabolites (Figure 7D). Spearman correlation analysis was performed to explore the potential association between the gut microbiota and metabolites. Lachnospiraceae are positively correlated with tryptophan, indole, and indole derivatives (supplemental Figure 6C-D), which was consistent with previous reports that Lachnospiraceae were involved in the production of tryptophan and indole.37
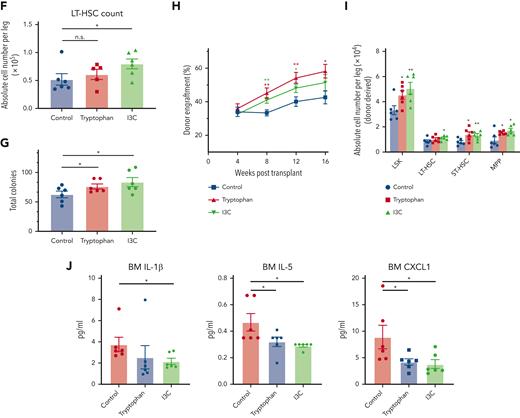
FMT-YA mice harbored a distinct metabolite landscape compared with FMT-AA mice. (A) PCA analysis to compare gut metabolome between FMT-AA and FMT-YA mice. (B) Volcano plot for differentially metabolites between FMT-AA and FMT-YA mice. (C) Heatmap showing the differentially produced metabolites between FMT-AA and FMT-YA mice. (D) Metabolite set enrichment of upregulated metabolites in FMT-YA mice compared with FMT-AA mice. (E) The flowchart of metabolite intervention. Aged mice were treated with different metabolites for 4 weeks and then the number and function of HSCs were evaluated. (F) Absolute numbers of LT-HSCs in BM in the control, tryptophan, and I3C groups. (G) Number of total colonies measured for LT-HSCs in methylcellulose. (H) Donor-derived CD45.2+ reconstitution in CD45.1+ mice at 16 weeks after HSC transplantation. (I) Absolute cell numbers of donor-derived HSPCs in BM from recipient mice in HSC transplantation assay. (J) Bar graphs showing the BM serum concentrations of IL-1β, IL-5, and CXCL1. Graphs show mean ± SEM, with statistical significance determined by Student t test (n = 6 per group). ∗P < .05, ∗∗P < .01. FC, fold change.
FMT-YA mice harbored a distinct metabolite landscape compared with FMT-AA mice. (A) PCA analysis to compare gut metabolome between FMT-AA and FMT-YA mice. (B) Volcano plot for differentially metabolites between FMT-AA and FMT-YA mice. (C) Heatmap showing the differentially produced metabolites between FMT-AA and FMT-YA mice. (D) Metabolite set enrichment of upregulated metabolites in FMT-YA mice compared with FMT-AA mice. (E) The flowchart of metabolite intervention. Aged mice were treated with different metabolites for 4 weeks and then the number and function of HSCs were evaluated. (F) Absolute numbers of LT-HSCs in BM in the control, tryptophan, and I3C groups. (G) Number of total colonies measured for LT-HSCs in methylcellulose. (H) Donor-derived CD45.2+ reconstitution in CD45.1+ mice at 16 weeks after HSC transplantation. (I) Absolute cell numbers of donor-derived HSPCs in BM from recipient mice in HSC transplantation assay. (J) Bar graphs showing the BM serum concentrations of IL-1β, IL-5, and CXCL1. Graphs show mean ± SEM, with statistical significance determined by Student t test (n = 6 per group). ∗P < .05, ∗∗P < .01. FC, fold change.
Next, we tested which metabolite played a role in the restorative effect of FMT. Aged mice were given standard diet supplemented with tryptophan, indole, IAA, I3C, or indole-3-butyric acid for 4 weeks (Figure 7E). Among these metabolites, only tryptophan and I3C enhanced the number of WBCs in BM, whereas the other interventions yielded weaker effects (supplemental Figure 6E-F). Strikingly, mice treated with I3C displayed an increased number of LT-HSCs in BM (Figure 7F; supplemental Figure 6G-J). Clonogenic assay and competitive repopulation assay of purified LT-HSCs showed that tryptophan and I3C improved clonogenic capacity and engraftment efficiency of LT-HSCs (Figure 7G-I; supplemental Figure 6K-M). Moreover, some inflammatory signals, including IL-1β, IL-5, and CXCL1, were significantly lower in BM serum of aged mice treated with tryptophan and I3C (Figure 7J). Collectively, these results indicate that FMT effectively reshaped the gut microbiota and metabolite landscape, which might underlie the restoration of aging hematopoiesis.
Discussion
In this study, we explored the roles of the gut microbiota in hematopoiesis in aged mice by using multiomic approaches, including scRNA-seq, 16S rRNA gene sequencing, and LC-MS untargeted metabolomic analysis. Our results showed that FMT from young mice could attenuate proinflammatory signaling, reverse myeloid-biased output, and rejuvenate aged HSCs. Lachnospiraceae were more abundant in FMT-YA mice, which improved both the number and the implantation ability of aged HSCs. Tryptophan metabolites were upregulated in FMT-YA mice and were associated with recovery of hematopoiesis. Together, these data indicated that gut microbiota from young mice and their metabolites could alleviate the detrimental effects on aged hematopoiesis.
The impact of gut microbiota on hematopoiesis have recently been reported.16-20 Depletion of gut microbiota suppressed the generation of peripheral lymphocytes and hematopoietic progenitors in BM.16,17,20 Moreover, microbiota played a vital role in HSC fate decisions by modulating distribution of iron.19 Our studies added to emerging evidence that gut microbiota from young mice could promote the recovery of aging hematopoiesis by reducing inflammatory signals. This restorative effect might be mediated through differential metabolites via gut microbiota–host interactions. Of note, the benefits of young gut microbiota are not limited to hematopoietic system but extend to facilitating germinal center formation in the gut,38 improving cognitive performance,39 and promoting poststroke recovery40,41 in aged mice.
scRNA-seq was applied to delineate the widespread transcriptional changes of HSCs as well as the microenvironment in which HSCs reside. Previous studies on HSC aging demonstrated that aged HSCs underwent fewer cell divisions because of an attenuation in cell cycle activity.42 Consistently, functional enrichment analysis of DEGs in HSCs showed that cell cycle–related genes were upregulated in FMT-YA mice, suggesting that young gut microbiota enhanced proliferation activity and improved self-renewal ability. Further analysis of cell-to-cell communications indicated that canonical inflammatory signals from myeloid cells toward HSCs were weakened in FMT-YA mice, including IL-1, IL-2, IL-17, and TNF. Interestingly, a recent study reported that gut microbiota affected the hematopoietic system via modulating the production of IL-1 and that knockout IL-1 receptor 1 led to mitigated inflammation-associated phenotypes in aging hematopoiesis.18 Furthermore, it is well known that aging is accompanied by chronic inflammation, and inflammageing provided the possibility of studying aging-related diseases from a promising viewpoint.43 However, eliminating harmful inflammaeging factors to prevent ineffectiveness of hematopoiesis remains challenging. Our study provided a novel insight for alleviating inflammaeging by FMT from young mice, which may lead to important progress in clinical application of FMT for antiaging. Furthermore, aging of the hematopoietic system is not only restricted to HSCs but also occurs in mature hematopoietic cells. For example, granulocytes displayed weaker migration ability in response to stimuli and macrophages exhibited impaired phagocytic activity during aging.43 Our scRNA-seq data implied that FMT from young mice could reverse senescent phenotype, enhance function of mature hematopoietic cells, and reinvigorate immunity.
Data from 16S rRNA gene sequencing indicated that Lachnospiraceae were more abundant in FMT-YA mice, and that mice supplied with Lachnospiraceae displayed an improvement in both the number and the function of aged HSCs. However, the roles of Lachnospiraceae on host health remains controversial.37 Lachnospiraceae could produce SCFAs that interact with the host immune system, reduce proinflammatory responses, restore hematopoiesis, and promote gastrointestinal repair after radiation.44-46 In clinical settings, abundance of Lachnospiraceae was associated with better survival outcome in patients with graft-versus-host disease after BM transplantation.47 On the contrary, Lachnospiraceae promoted the onset of diabetes by impairing glucose metabolism46 and was associated with declining renal function.48 Furthermore, the abundance of several taxa of Lachnospiraceae was positively correlated with major depressive disorder and stress-induced behavioral changes in patients.48-50 This controversy may be attributed to the limited understanding of subpopulations of Lachnospiraceae because of potential limitations of identification and purification of these bacteria. Although our study demonstrated the beneficial effects of Lachnospiraceae in the recovery of hematopoiesis, further studies are warranted to discern the contributing genus and the specific mechanisms involved in the interactions between Lachnospiraceae and the host.
Looking into the underlying mechanisms of the effects of young gut microbiota on hematopoiesis, we found that FMT altered tryptophan-related metabolites in the intestinal microenvironment, which led to the accumulation of indole and indole derivatives in FMT-YA mice. Tryptophan and its metabolites are important regulators of gut homeostasis, and dietary deficiency in tryptophan leads to gut microbiota dysbiosis and systemic inflammation.51,52 Consistently, a tryptophan metabolite, IAA, contributed to the scavenging of free radicals36 and attenuated oxidative stress and inflammatory stress induced by a high-fat diet.35 Our results demonstrated that tryptophan-related metabolites played a role in the restorative effect of young gut microbiota. Furthermore, several prominent metabolites with anti-inflammatory activities were identified by LC-MS untargeted metabolomic analysis and may explain the improvement in the hematopoietic system in aged mice. Enterolactone, an antioxidative organic compound derived from flaxseed, has been reported to protect intestinal barrier integrity from inflammation by reducing oxidative stress damage.53,54 Moreover, fatty acid esters of hydroxy fatty acids have been shown to have anti-inflammatory effects both in vivo and in vitro.55 Although our data show changes in metabolites after FMT and provide rational for further antisenescence metabolite screening, further studies are required to investigate specific functions of these metabolites and their underlying molecular mechanisms in regulating hematopoiesis.
With the accumulation of evidence explaining potential effects of gut microbiota on hematopoiesis, a “gut-BM axis” has been proposed as a novel player in HSC aging.18,56 In summary, our study provides a broader view of the mechanisms of the microbiota-gut-BM axis and indicates that manipulating the gut ecosystem may be a promising strategy to rejuvenate aged HSCs. Nevertheless, we believe that this warrants future investigation to elucidate the relationship between gut microbiota and development of clonal hematopoiesis in humans.
Acknowledgments
The authors express their gratitude for the support and help from members of the staff of the core facility platform of the Zhejiang University School of Medicine and the Laboratory Animal Center of Zhejiang University.
This work was supported by grants from the National Key Research and Development Program of China (2022YFA1103500 and 2018YFA0109300), the National Natural Science Foundation of China (82130003, 82222003, 92268117, 81870080, 91949115, 82161138028, 82070179, and 82000179), the Key R&D project of Zhejiang Provincial Science and Technology Department (2021C03010), the Zhejiang Provincial Natural Science Foundation of China (LY21H080004 and LR19H080001), the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (2020R01006), and Research Center of Prevention and Treatment of Senescence Syndrome, School of Medicine Zhejiang University, 2022020002.
Authorship
Contribution: P.Q., H.H., X.Z., and Xiaoqing Li conceived the study; X.Z., Xiaoqing Li, Xia Li, C.W., and C.S. curated and analyzed the data; P.Q. and H.H. determined the methodology and acquired funding; X.Z. and Xiaoqing Li wrote the original draft; and K.H., D.K., Q.L., Y.X., W.S., M.Z., J.S., J.F., Y.H., H.H., and P.Q. reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pengxu Qian, School of Medicine, Zhejiang University, Room 209, Zijingang Campus, No. 866 Yuhangtang Rd, Hangzhou 310030, China; e-mail: axu@zju.edu.cn; and He Huang, Bone Marrow Transplantation Center, The First Affiliated Hospital, School of Medicine, Zhejiang University, No. 79 Qingchun Rd, Hangzhou 310003, China; e-mail: huanghe@zju.edu.cn.
References
Author notes
∗X.Z., Xiaoqing Li, Xia Li, C.W., and C.S. contributed equally to this study.
The data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE201920) and the Sequence Read Archive database (accession number PRJNA832671).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal