Abstract
Background: FRSW 107, a novel homodimer recombinant factor VIII (rFVIII) Fc fusion protein, is the first domestic extended half-life (EHL) rFVIII under development in China,and is currently at stage of Biologics License Application (BLA) in NMPA for the prophylaxis and treatment of bleeding episodes in adolescent and adult (≥12 yr) with hemophilia A (HA).
Aims: This phase III (NCT04456387, CTR20201212), open-label, non-randomized, multicenter trial aimed to evaluate the pharmacokinetics (PK), safety, and clinical efficacy of FRSW 107 for prophylaxis and treatment of bleeding in previously treated HA patients ≥12 yr.
Methods: Eligible patients included male severe HA (FVIII:C< 1%) ≥12 yr, previous treatment with FVIII concentrates for ≥150 exposure days (EDs), and absence of FVIII inhibitors (<0.6 Bethesda Units/mL). A total of 119 patients received FRSW 107 for prophylaxis treatment (n=83) or on-demand treatment (n=36). The patients in the prophylaxis group were administered one dose of 50 IU/kg of FRSW 107 every three days for at least 50 EDs for a minimum period of 6 months, and the primary efficacy endpoint was annualized bleeding rate (ABR). The patients in the on-demand treatment group received 30 to 50 IU/kg of FRSW 107 over 6 months, according to the severity and location of the bleeding, and the primary efficacy endpoint was hemostatic effect (excellent, good). The single-dose and repeat dose PK parameters of 15 patients in the prophylaxis group were analyzed at ED1 and ED35, respectively.
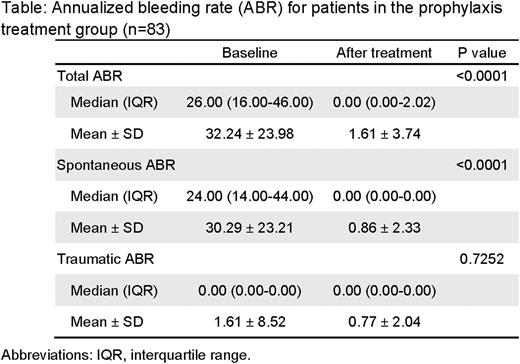
Results: In the prophylaxis group, the mean and median annualized bleeding rate (ABR) were 1.61±3.74 and 0.00 (IQR, 0.00-2.02), decreased by 95.01% versus the baseline 32.24±23.98 and 26.00 (IQR, 16.00-46.00), and 56 (67.5%) patients had no bleeds during the trial. Detailed ABR data for prophylaxis group was included in the table below. In the on-demand treatment group, hemostatic efficacy was rated as "excellent” or "good” for 98.9% of bleeds. 97.9% of 721 reported bleeds were treated with ≤2 injections. No FVIII inhibitors have been reported in this study. Drug-related adverse events (AEs) occurred in 10.1% of the patients. No SAE was judged to be related to FRSW107 treatment. No thromboembolic events were reported, and there are no unexpected safety concerns. The terminal half-life of FRSW 107 ranged between 20 to 22 hours. The incremental recovery (IR) was approximately 2.14 (IU/dL)/(IU/kg). The PK parameters and profiles remained consistent between ED1 and the repeated dosing at ED35.
Conclusions: The data indicate that FRSW 107 was safe and effective for prophylaxis and treatment of bleeding episodes in previously treated adolescent and adult HA patients.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal